Stimulation of phosphatidylinositol 3-kinase by fibroblast growth factor receptors is mediated by coordinated recruitment of multiple docking proteins
- PMID: 11353842
- PMCID: PMC33424
- DOI: 10.1073/pnas.111114298
Stimulation of phosphatidylinositol 3-kinase by fibroblast growth factor receptors is mediated by coordinated recruitment of multiple docking proteins
Abstract
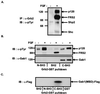
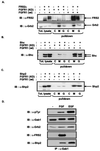
The docking protein FRS2 is a major downstream effector that links fibroblast growth factor (FGF) and nerve growth factor receptors with the Ras/mitogen-activated protein kinase signaling cascade. In this report, we demonstrate that FRS2 also plays a pivotal role in FGF-induced recruitment and activation of phosphatidylinositol 3-kinase (PI3-kinase). We demonstrate that tyrosine phosphorylation of FRS2alpha leads to Grb2-mediated complex formation with the docking protein Gab1 and its tyrosine phosphorylation, resulting in the recruitment and activation of PI3-kinase. Furthermore, Grb2 bound to tyrosine-phosphorylated FRS2 through its SH2 domain interacts primarily via its carboxyl-terminal SH3 domain with a proline-rich region in Gab1 and via its amino-terminal SH3 domain with the nucleotide exchange factor Sos1. Assembly of FRS2alpha:Grb2:Gab1 complex induced by FGF stimulation results in activation of PI3-kinase and downstream effector proteins such as the S/T kinase Akt, whose cellular localization and activity are regulated by products of PI3-kinase. These experiments reveal a unique mechanism for generation of signal diversity by growth factor-induced coordinated assembly of a multidocking protein complex that can activate the Ras/mitogen-activated protein kinase cascade to induce cell proliferation and differentiation, and PI3-kinase to activate a mediator of a cell survival pathway.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

