Membrane resonance and subthreshold membrane oscillations in mesencephalic V neurons: participants in burst generation
- PMID: 11356860
- PMCID: PMC6762704
- DOI: 10.1523/JNEUROSCI.21-11-03729.2001
Membrane resonance and subthreshold membrane oscillations in mesencephalic V neurons: participants in burst generation
Abstract
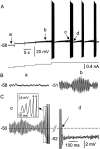
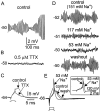
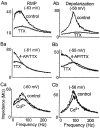
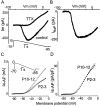
Trigeminal mesencephalic (Mes V) neurons are critical components of the circuits controlling oral-motor activity. The possibility that they can function as interneurons necessitates a detailed understanding of the factors controlling their soma excitability. Using whole-cell patch-clamp recording, in vitro, we investigated the development of the ionic mechanisms responsible for the previously described subthreshold membrane oscillations and rhythmical burst discharge in Mes V neurons from rats ages postnatal day (P) 2-12. We found that the oscillation amplitude and frequency increased during development, whereas bursting emerged after P6. Furthermore, when bursting was initiated, the spike frequency was largely determined by the oscillation frequency. Frequency domain analysis indicated that these oscillations emerged from the voltage-dependent resonant properties of Mes V neurons. Low doses of 4-aminopyridine (<100 microm) reduced the oscillations and abolished resonance in most neurons, suggesting that the resonant current is a steady-state K(+) current (I(4-AP)). Sodium ion replacement or TTX reduced substantially the oscillations and peak amplitude of the resonance, suggesting the presence of a persistent Na(+) current (I(NaP)) that functions to amplify the resonance and facilitate the emergence of subthreshold oscillations and bursting.
Figures





Similar articles
-
Persistent sodium currents in mesencephalic v neurons participate in burst generation and control of membrane excitability.J Neurophysiol. 2005 May;93(5):2710-22. doi: 10.1152/jn.00636.2004. Epub 2004 Dec 29. J Neurophysiol. 2005. PMID: 15625100
-
Resonance characteristic and its ionic basis of rat mesencephalic trigeminal neurons.Brain Res. 2015 Jan 30;1596:1-12. doi: 10.1016/j.brainres.2014.10.064. Epub 2014 Nov 6. Brain Res. 2015. PMID: 25449887
-
Participation of Kv1 channels in control of membrane excitability and burst generation in mesencephalic V neurons.J Neurophysiol. 2009 Mar;101(3):1407-18. doi: 10.1152/jn.91053.2008. Epub 2009 Jan 14. J Neurophysiol. 2009. PMID: 19144742 Free PMC article.
-
Participation of sodium currents in burst generation and control of membrane excitability in mesencephalic trigeminal neurons.J Neurosci. 2006 Mar 29;26(13):3412-22. doi: 10.1523/JNEUROSCI.5274-05.2006. J Neurosci. 2006. PMID: 16571748 Free PMC article.
-
Subthreshold membrane resonance in neocortical neurons.J Neurophysiol. 1996 Aug;76(2):683-97. doi: 10.1152/jn.1996.76.2.683. J Neurophysiol. 1996. PMID: 8871191
Cited by
-
Modulation of SK Channels via Calcium Buffering Tunes Intrinsic Excitability of Parvalbumin Interneurons in Neuropathic Pain: A Computational and Experimental Investigation.J Neurosci. 2023 Aug 2;43(31):5608-5622. doi: 10.1523/JNEUROSCI.0426-23.2023. Epub 2023 Jul 14. J Neurosci. 2023. PMID: 37451982 Free PMC article.
-
Ectopic discharge in Abeta afferents as a source of neuropathic pain.Exp Brain Res. 2009 Jun;196(1):115-28. doi: 10.1007/s00221-009-1724-6. Epub 2009 Feb 26. Exp Brain Res. 2009. PMID: 19242687 Review.
-
Spiking resonances in models with the same slow resonant and fast amplifying currents but different subthreshold dynamic properties.J Comput Neurosci. 2017 Dec;43(3):243-271. doi: 10.1007/s10827-017-0661-9. Epub 2017 Oct 24. J Comput Neurosci. 2017. PMID: 29064059
-
Different species, different gap junctions?J Gen Physiol. 2023 Sep 4;155(9):e202313430. doi: 10.1085/jgp.202313430. Epub 2023 Aug 1. J Gen Physiol. 2023. PMID: 37526640 Free PMC article.
-
Comparative study between radiofrequency-induced and muscimol-induced inhibition of cultured networks of cortical neuron.PLoS One. 2022 Aug 31;17(8):e0268605. doi: 10.1371/journal.pone.0268605. eCollection 2022. PLoS One. 2022. PMID: 36044461 Free PMC article.
References
-
- Alley KE. Quantitative analysis of the synaptogenic period in the trigeminal mesencephalic nucleus. Anat Rec. 1973;177:49–59. - PubMed
-
- Buisseret-Delmas C, Pinganaud G, Compoint C, Buisseret P. Projection from trigeminal nuclei to neurons of the mesencephalic trigeminal nucleus in rat. Neurosci Lett. 1997;229:189–192. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
