Temporary inactivation of the retrosplenial cortex causes a transient reorganization of spatial coding in the hippocampus
- PMID: 11356886
- PMCID: PMC6762703
- DOI: 10.1523/JNEUROSCI.21-11-03986.2001
Temporary inactivation of the retrosplenial cortex causes a transient reorganization of spatial coding in the hippocampus
Abstract
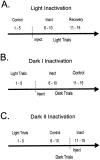
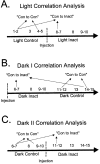
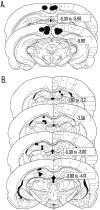
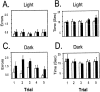
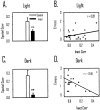
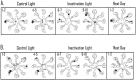
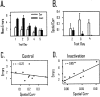
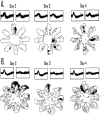
The ability to navigate accurately is dependent on the integration of visual and movement-related cues. Navigation based on metrics derived from movement is referred to as path integration. Recent theories of navigation have suggested that posterior cortical areas, the retrosplenial and posterior parietal cortex, are involved in path integration during navigation. In support of this hypothesis, we have found previously that temporary inactivation of retrosplenial cortex results in dark-selective impairments on the radial maze (Cooper and Mizumori, 1999). To understand further the role of the retrosplenial cortex in navigation, we combined temporary inactivation of retrosplenial cortex with recording of complex spike cells in the hippocampus. Thus, behavioral performance during spatial memory testing could be compared with place-field responses before, and during, inactivation of retrosplenial cortex. In the first experiment, behavioral results confirmed that inactivation of retrosplenial cortex only impairs radial maze performance in darkness when animals are at asymptote levels of performance. A second experiment revealed that retrosplenial cortex inactivation impaired spatial learning during initial light training. In both experiments, the normal location of hippocampal "place fields" was changed by temporary inactivation of retrosplenial cortex, whereas other electrophysiological properties of the cells were not affected. The changes in place coding occurred in the presence, and absence, of behavioral impairments. We suggest that the retrosplenial cortex provides mnemonic spatial information for updating location codes in the hippocampus, thereby facilitating accurate path integration. In this way, the retrosplenial cortex and hippocampus may be part of an interactive neural system that mediates navigation.
Figures


Similar articles
-
Retrosplenial cortex inactivation selectively impairs navigation in darkness.Neuroreport. 1999 Feb 25;10(3):625-30. doi: 10.1097/00001756-199902250-00033. Neuroreport. 1999. PMID: 10208601
-
Retrosplenial cortex lesions impair water maze strategies learning or spatial place learning depending on prior experience of the rat.Behav Brain Res. 2006 Jun 30;170(2):316-25. doi: 10.1016/j.bbr.2006.03.003. Epub 2006 Apr 18. Behav Brain Res. 2006. PMID: 16621053
-
Encoding and storage of spatial information in the retrosplenial cortex.Proc Natl Acad Sci U S A. 2014 Jun 10;111(23):8661-6. doi: 10.1073/pnas.1313222111. Epub 2014 May 27. Proc Natl Acad Sci U S A. 2014. PMID: 24912150 Free PMC article.
-
The separate and combined properties of the granular (area 29) and dysgranular (area 30) retrosplenial cortex.Neurobiol Learn Mem. 2021 Nov;185:107516. doi: 10.1016/j.nlm.2021.107516. Epub 2021 Sep 3. Neurobiol Learn Mem. 2021. PMID: 34481970 Review.
-
Parallel processing of information about location in the amygdala, entorhinal cortex and hippocampus.Hippocampus. 2013 Nov;23(11):1075-83. doi: 10.1002/hipo.22179. Hippocampus. 2013. PMID: 23929819 Review.
Cited by
-
Retrosplenial and subicular inputs converge on superficially projecting layer V neurons of medial entorhinal cortex.Brain Struct Funct. 2022 Nov;227(8):2821-2837. doi: 10.1007/s00429-022-02578-8. Epub 2022 Oct 14. Brain Struct Funct. 2022. PMID: 36229654 Free PMC article.
-
The Signature of Moderate Perinatal Hypoxia on Cortical Organization and Behavior: Altered PNN-Parvalbumin Interneuron Connectivity of the Cingulate Circuitries.Front Cell Dev Biol. 2022 Feb 28;10:810980. doi: 10.3389/fcell.2022.810980. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 35295859 Free PMC article.
-
Retrosplenial Cortex Effects Contextual Fear Formation Relying on Dysgranular Constituent in Rats.Front Neurosci. 2022 May 3;16:886858. doi: 10.3389/fnins.2022.886858. eCollection 2022. Front Neurosci. 2022. PMID: 35592254 Free PMC article.
-
Homeostatic regulation of memory systems and adaptive decisions.Hippocampus. 2013 Nov;23(11):1103-24. doi: 10.1002/hipo.22176. Hippocampus. 2013. PMID: 23929788 Free PMC article. Review.
-
Knockout mice reveal a role for protein tyrosine phosphatase H1 in cognition.Behav Brain Funct. 2008 Aug 12;4:36. doi: 10.1186/1744-9081-4-36. Behav Brain Funct. 2008. PMID: 18700002 Free PMC article.
References
-
- Alyan S, McNaughton BL. Hippocampectomized rats are capable of homing by path integration. Behav Neurosci. 1999;113:19–31. - PubMed
-
- Barnes CA, Suster MS, Shen J, McNaughton BL. Multistability of cognitive maps in the hippocampus of old rats. Nature. 1997;388:272–275. - PubMed
-
- Blair HT, Sharp PE. Visual and vestibular influences on head-direction cells in the anterior thalamus of the rat. Behav Neurosci. 1996;110:643–660. - PubMed
-
- Brown MF, Bing MN. In the dark: spatial choice when access to spatial cues is restricted. Anim Learn Behav. 1997;25:21–30.
-
- Chen LL, Lin LH, Green EJ, Barnes CA, McNaughton BL. Head-direction cells in the rat posterior cortex. I. Anatomical distribution and behavioral modulation. Exp Brain Res. 1994a;101:8–23. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
