Neurons of a limited subthalamic area mediate elevations in cortical cerebral blood flow evoked by hypoxia and excitation of neurons of the rostral ventrolateral medulla
- PMID: 11356890
- PMCID: PMC6762684
- DOI: 10.1523/JNEUROSCI.21-11-04032.2001
Neurons of a limited subthalamic area mediate elevations in cortical cerebral blood flow evoked by hypoxia and excitation of neurons of the rostral ventrolateral medulla
Abstract
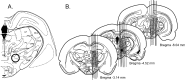
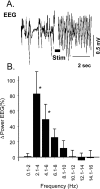
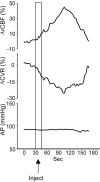
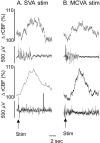
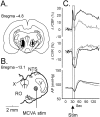
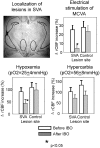
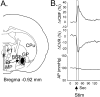
Sympathoexcitatory reticulospinal neurons of the rostral ventrolateral medulla (RVLM) are oxygen detectors excited by hypoxia to globally elevate regional cerebral blood flow (rCBF). The projection, which accounts for >50% of hypoxic cerebral vasodilation, relays through the medullary vasodilator area (MCVA). However, there are no direct cortical projections from the RVLM/MCVA, suggesting a relay that diffusely innervates cortex and possibly originates in thalamic nuclei. Systematic mapping by electrical microstimulation of the thalamus and subthalamus revealed that elevations in rCBF were elicited only from a limited area, which encompassed medial pole of zona incerta, Forel's field, and prerubral zone. Stimulation (10 sec train) at an active site increased rCBF by 25 +/- 6%. Excitation of local neurons with kainic acid mimicked effects of electrical stimulation by increasing rCBF. Stimulation of the subthalamic cerebrovasodilator area (SVA) with single pulses (0.5 msec; 80 microA) triggered cortical EEG burst-CBF wave complexes with latency 24 +/- 5 msec, which were similar in shape to complexes evoked from the MCVA. Selective bilateral lesioning of the SVA neurons (ibotenic acid, 2 microg, 200 nl) blocked the vasodilation elicited from the MCVA and attenuated hypoxic cerebrovasodilation by 52 +/- 12% (p < 0.05), whereas hypercarbic vasodilation remained preserved. Lesioning of the vasodilator site in the basal forebrain failed to modify SVA-evoked rCBF increase. We conclude that (1) excitation of intrinsic neurons of functionally restricted region of subthalamus elevates rCBF, (2) these neurons relay signals from the MCVA, which elevate rCBF in response to hypoxia, and (3) the SVA is a functionally important site conveying vasodilator signal from the medulla to the telencephalon.
Figures

Similar articles
-
A brainstem area mediating cerebrovascular and EEG responses to hypoxic excitation of rostral ventrolateral medulla in rat.J Physiol. 2000 Dec 1;529 Pt 2(Pt 2):413-29. doi: 10.1111/j.1469-7793.2000.00413.x. J Physiol. 2000. PMID: 11101651 Free PMC article.
-
The medullary cerebrovascular vasodilator area mediates cerebrovascular vasodilation and electroencephalogram synchronization elicited from cerebellar fastigial nucleus in Sprague-Dawley rats.Neurosci Lett. 2000 Jul 21;288(3):183-6. doi: 10.1016/s0304-3940(00)01228-3. Neurosci Lett. 2000. PMID: 10889338
-
Neurons of nucleus of the solitary tract synchronize the EEG and elevate cerebral blood flow via a novel medullary area.Brain Res. 2001 Feb 16;892(1):1-12. doi: 10.1016/s0006-8993(00)02949-8. Brain Res. 2001. PMID: 11172744
-
Sympatho-excitatory neurons of the rostral ventrolateral medulla are oxygen sensors and essential elements in the tonic and reflex control of the systemic and cerebral circulations.J Hypertens Suppl. 1994 Dec;12(10):S159-80. J Hypertens Suppl. 1994. PMID: 7769486 Review.
-
Central neurogenic neuroprotection: central neural systems that protect the brain from hypoxia and ischemia.Ann N Y Acad Sci. 1997 Dec 19;835:168-86. doi: 10.1111/j.1749-6632.1997.tb48628.x. Ann N Y Acad Sci. 1997. PMID: 9616772 Review.
Cited by
-
Neurogenic neuroprotection: clinical perspectives.Funct Neurol. 2012 Oct-Dec;27(4):207-16. Funct Neurol. 2012. PMID: 23597434 Free PMC article.
-
Alterations in cerebral blood flow and cerebrovascular reactivity during 14 days at 5050 m.J Physiol. 2011 Feb 1;589(Pt 3):741-53. doi: 10.1113/jphysiol.2010.192534. Epub 2010 Nov 1. J Physiol. 2011. PMID: 21041534 Free PMC article. Clinical Trial.
-
Neuroprotection during Thrombectomy for Acute Ischemic Stroke: A Review of Future Therapies.Int J Mol Sci. 2024 Jan 10;25(2):891. doi: 10.3390/ijms25020891. Int J Mol Sci. 2024. PMID: 38255965 Free PMC article. Review.
-
Research progress on the mechanism of cerebral blood flow regulation in hypoxia environment at plateau.Bioengineered. 2022 Mar;13(3):6353-6358. doi: 10.1080/21655979.2021.2024950. Bioengineered. 2022. PMID: 35235760 Free PMC article. Review.
-
Essential role of hemoglobin beta-93-cysteine in posthypoxia facilitation of breathing in conscious mice.J Appl Physiol (1985). 2014 May 15;116(10):1290-9. doi: 10.1152/japplphysiol.01050.2013. Epub 2014 Mar 7. J Appl Physiol (1985). 2014. PMID: 24610531 Free PMC article.
References
-
- Amzica F, Steriade M. Electrophysiological correlates of sleep delta waves. Electroencephalogr Clin Neurophysiol. 1998;107:69–83. - PubMed
-
- Biesold D, Inanami O, Sato A, Sato Y. Stimulation of the nucleus basalis of Meynert increases cerebral cortical blood flow in rats. Neurosci Lett. 1989;98:39–44. - PubMed
-
- Blix AS, Folkow B. Cardiovascular adjustments to diving in mammals and birds. In: Renkin EM, Michel CC, editors. The cardiovascular system, Section 2. American Physiological Society; Bethesda, MD: 1983. pp. 917–945.
-
- Butler PJ. Respiratory and cardiovascular control during diving in birds and mammals. J Exp Biol. 1982;100:195–221. - PubMed
-
- Dampney RA, Moon EA. Role of ventrolateral medulla in vasomotor response to cerebral ischemia. Am J Physiol. 1980;239:H349–358. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
