Tonic control of peripheral cutaneous nociceptors by somatostatin receptors
- PMID: 11356891
- PMCID: PMC6762714
- DOI: 10.1523/JNEUROSCI.21-11-04042.2001
Tonic control of peripheral cutaneous nociceptors by somatostatin receptors
Abstract
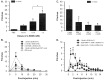
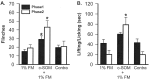
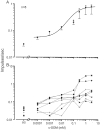
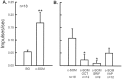
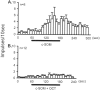
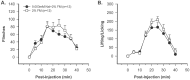
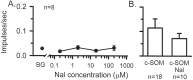
The peptide somatostatin [somatotropin release-inhibiting factor (SRIF)] is widely distributed in the body and exerts a variety of hormonal and neural actions. Several lines of evidence indicate that SRIF is important in nociceptive processing: (1) it is localized in a subset of small-diameter dorsal root ganglion cells; (2) activation of SRIF receptors results in inhibition of both nociceptive behaviors in animals and acute and chronic pain in humans; (3) SRIF inhibits dorsal horn neuronal activity; and (4) SRIF reduces responses of joint mechanoreceptors to noxious rotation of the knee joint. The goal of the present study is to show that cutaneous nociceptors are under the tonic inhibitory control of SRIF. This is accomplished using behavioral and electrophysiological paradigms. In a dose-dependent manner, intraplantar injection of the SRIF receptor antagonist cyclo-somatostatin (c-SOM) results in nociceptive behaviors in normal animals and enhancement of nociceptive behaviors in formalin-injected animals, and these actions can be blocked when c-SOM is coapplied with three different SRIF agonists. Furthermore, intraplantar injection of SRIF antiserum also results in nociceptive behaviors. Electrophysiological recordings using an in vitro glabrous skin-nerve preparation show increased nociceptor activity in response to c-SOM, and this increase is blocked by the same three SRIF agonists. Parallel behavioral and electrophysiological studies using the opioid antagonist naloxone demonstrate that endogenous opioids do not maintain a tonic inhibitory control over peripheral nociceptors, nor does opioid receptor antagonism influence peripheral SRIF effects on nociceptors. These findings demonstrate that SRIF receptors maintain a tonic inhibitory control over peripheral nociceptors, and this may contribute to mechanisms that control the excitability of these terminals.
Figures



References
-
- Carlton SM, Zhou S, Coggeshall RE. Peripheral GABAA receptors: evidence for peripheral primary afferent depolarization. Neuroscience. 1999;93:713–722. - PubMed
-
- Carlton SM, Du J, Davidson E, Zhou S, Coggeshall RE. Somatostatin receptors on peripheral primary afferent terminals: inhibition of sensitized nociceptors. Pain. 2001;90:233–244. - PubMed
-
- Chapman V, Dickenson AH. The effects of sandostatin and somatostatin on nociceptive transmission in the dorsal horn of the rat spinal cord. Neuropeptides. 1992;23:147–152. - PubMed
-
- Chatila R, Ferayorni L, Gupta T, Groszmann RJ. Local arterial vasoconstriction induced by octreotide in patients with cirrhosis. Hepatology. 2000;31:572–576. - PubMed
-
- Chrubasik J, Meynadier J, Blond S, Scherpereel P, Ackerman E, Weinstock M, Bonath K, Cramer H, Wunsch E. Somatostatin, a potent analgesic. Lancet. 1984;2:1208–1209. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
