Dopamine attenuates prefrontal cortical suppression of sensory inputs to the basolateral amygdala of rats
- PMID: 11356897
- PMCID: PMC6762693
- DOI: 10.1523/JNEUROSCI.21-11-04090.2001
Dopamine attenuates prefrontal cortical suppression of sensory inputs to the basolateral amygdala of rats
Abstract
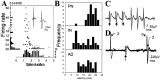
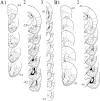
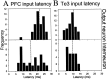
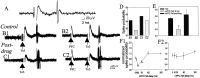
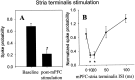
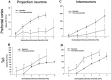
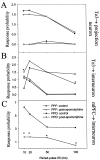
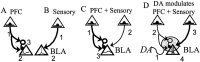
The basolateral complex of the amygdala (BLA) plays a significant role in affective behavior that is likely regulated by afferents from the medial prefrontal cortex (mPFC). Studies suggest that dopamine (DA) is a necessary component for production of appropriate affective responses. In this study, prefrontal cortical and sensory cortical [temporal area 3 (Te3)] inputs to the BLA and their modulation by DA receptor activation was examined using in vivo single-unit extracellular recordings. We found that Te3 inputs are more capable of driving BLA projection neuron firing, whereas mPFC inputs potently elicited firing from BLA interneurons. Moreover, mPFC stimulation before Te3 stimulation attenuated the probability of Te3-evoked spikes in BLA projection neurons, possibly via activation of inhibitory interneurons. DA receptor activation by apomorphine attenuated mPFC inputs, while augmenting Te3 inputs. Additionally, DA receptor activation suppressed mPFC-induced inhibition of Te3-evoked spikes. Thus, the mPFC may attenuate sensory-driven amygdala-mediated affective responses via recruitment of BLA inhibitory interneurons that suppress sensory cortical inputs. In situations of enhanced DA levels in the BLA, such as during stress and after amphetamine administration, mPFC regulation of BLA will be dampened, leading to a disinhibition of sensory-driven affective responses.
Figures




References
-
- Al Maskati HA, Zbrozyna AW. Stimulation in prefrontal cortex area inhibits cardiovascular and motor components of the defence reaction in rats. J Auton Nerv Syst. 1989;28:117–126. - PubMed
-
- Alheid G, de Olmos JS, Beltramino CA. Amygdala and extended amygdala. In: Paxinos G, editor. The rat nervous system, Ed 2. Academic; Sydney: 1995. pp. 495–572.
-
- Andreasen NC, O'Leary DS, Flaum M, Nopoulos P, Watkins GL, Boles Ponto LL, Hichwa RD. Hypofrontality in schizophrenia: distributed dysfunctional circuits in neuroleptic-naive patients. Lancet. 1997;349:1730–1734. - PubMed
-
- Armony JL, Servan-Schreiber D, Romanski LM, Cohen JD, LeDoux JE. Stimulus generalization of fear responses: effects of auditory cortex lesions in a computational model and in rats. Cereb Cortex. 1997;7:157–165. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
