Postentry restriction to human immunodeficiency virus-based vector transduction in human monocytes
- PMID: 11356951
- PMCID: PMC114256
- DOI: 10.1128/JVI.75.12.5448-5456.2001
Postentry restriction to human immunodeficiency virus-based vector transduction in human monocytes
Abstract
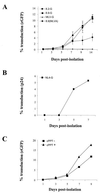
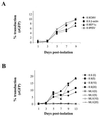
Cells of the monocyte lineage can be infected with human immunodeficiency virus type 1 (HIV-1) both during clinical infection and in vitro. The ability of HIV-1-based vectors to transduce human monocytes, monocyte-derived macrophages, and dendritic cells (DCs) was therefore examined, in order to develop an efficient protocol for antigen gene delivery to human antigen-presenting cells. Freshly isolated monocytes were refractory to HIV-1-based vector transduction but became transducible after in vitro differentiation to mature macrophages. This maturation-dependent transduction was independent of the HIV-1 accessory proteins Vif, Vpr, Vpu, and Nef in the packaging cells and of the central polypurine tract in the vector, and it was also observed with a vesicular stomatitis virus-pseudotyped HIV-1 provirus, defective only in envelope and Nef. The level and extent of reverse transcription of the HIV-1-based vector was similar after infection of immature monocytes and of mature macrophages. However, 2LTR vector circles could not be detected in monocytes, suggesting a block to vector nuclear entry in these cells. Transduction of freshly isolated monocytes exposed to HIV-1-based vector could be rescued by subsequent differentiation into DCs. This rescue was induced by fetal calf serum in the DC culture medium, which promoted vector nuclear entry.
Figures





Similar articles
-
Transduction of human PBMC-derived dendritic cells and macrophages by an HIV-1-based lentiviral vector system.Mol Ther. 2000 Feb;1(2):171-9. doi: 10.1006/mthe.2000.0027. Mol Ther. 2000. PMID: 10933928
-
HIV-1 Vpr inhibits the maturation and activation of macrophages and dendritic cells in vitro.Int Immunol. 2005 Feb;17(2):103-16. doi: 10.1093/intimm/dxh190. Epub 2004 Dec 20. Int Immunol. 2005. PMID: 15611322
-
Comparison between Sendai virus and adenovirus vectors to transduce HIV-1 genes into human dendritic cells.J Med Virol. 2008 Mar;80(3):373-82. doi: 10.1002/jmv.21052. J Med Virol. 2008. PMID: 18205221
-
Regulation of chemokine/cytokine network during in vitro differentiation and HIV-1 infection of human monocytes: possible importance in the pathogenesis of AIDS.J Leukoc Biol. 2000 Sep;68(3):391-9. J Leukoc Biol. 2000. PMID: 10985256 Review.
-
Regulation of HIV-1 transcription in cells of the monocyte-macrophage lineage.Retrovirology. 2009 Dec 23;6:118. doi: 10.1186/1742-4690-6-118. Retrovirology. 2009. PMID: 20030845 Free PMC article. Review.
Cited by
-
Interaction of Vpx and apolipoprotein B mRNA-editing catalytic polypeptide 3 family member A (APOBEC3A) correlates with efficient lentivirus infection of monocytes.J Biol Chem. 2010 Apr 16;285(16):12248-54. doi: 10.1074/jbc.M109.090977. Epub 2010 Feb 23. J Biol Chem. 2010. PMID: 20178977 Free PMC article.
-
Multiple restrictions of human immunodeficiency virus type 1 in feline cells.J Virol. 2007 Jul;81(13):7048-60. doi: 10.1128/JVI.02714-06. Epub 2007 Apr 25. J Virol. 2007. PMID: 17459941 Free PMC article.
-
Transduction efficiency of MLV but not of HIV-1 vectors is pseudotype dependent on human primary T lymphocytes.J Mol Med (Berl). 2003 Dec;81(12):801-10. doi: 10.1007/s00109-003-0491-2. Epub 2003 Oct 24. J Mol Med (Berl). 2003. PMID: 14576928
-
HIV Replication in Humanized IL-3/GM-CSF-Transgenic NOG Mice.Pathogens. 2019 Mar 12;8(1):33. doi: 10.3390/pathogens8010033. Pathogens. 2019. PMID: 30871027 Free PMC article.
-
Circulating monocytes are not a major reservoir of HIV-1 in elite suppressors.J Virol. 2011 Oct;85(19):10399-403. doi: 10.1128/JVI.05409-11. Epub 2011 Jul 27. J Virol. 2011. PMID: 21795348 Free PMC article.
References
-
- Ayehunie S, Garcia-Zepeda E, Hoxie J, Horuk R, Kupper T, Luster A, Ruprecht R. Human immunodeficiency virus-1 entry into purified blood dendritic cells through CC and CXC chemokine coreceptors. Blood. 1997;90:1379–1386. - PubMed
-
- Banchereau J, Steinman R M. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. - PubMed
-
- Briant L, Robert-Hebmann V, Acquaviva C, Pelchen-Matthews A, Marsh M, Devaux C. The protein tyrosine kinase p56lck is required for triggering NF-kappaB activation upon interaction of human immunodeficiency virus type 1 envelope glycoprotein gp120 with cell surface CD4. J Virol. 1998;72:6207–6214. - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

