Telomerase activation by human papillomavirus type 16 E6 protein: induction of human telomerase reverse transcriptase expression through Myc and GC-rich Sp1 binding sites
- PMID: 11356963
- PMCID: PMC114268
- DOI: 10.1128/JVI.75.12.5559-5566.2001
Telomerase activation by human papillomavirus type 16 E6 protein: induction of human telomerase reverse transcriptase expression through Myc and GC-rich Sp1 binding sites
Abstract
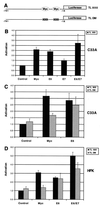
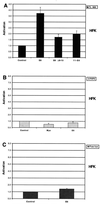
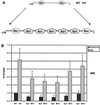
High-risk human papillomaviruses (HPVs) immortalize keratinocytes by disrupting the retinoblastoma protein (Rb)/p16 pathway and activating telomerase. The E7 oncoprotein targets Rb, while the E6 oncoprotein induces telomerase activity in human keratinocytes. This study has examined the mechanism by which E6 activates telomerase. Expression of human telomerase reverse transcriptase (hTERT), the catalytic subunit of telomerase, was found to be increased in keratinocytes stably expressing HPV type 16 E6, suggesting that E6 acts to increase hTERT transcription. hTERT expression and telomerase activity were activated to significantly higher levels in cells expressing both E6 and E7 than in cells expressing E6 alone. This indicates that E7 may augment E6-mediated activation of hTERT transcription. In transient-transfection assays using hTERT reporters, the induction of hTERT expression by E6 was found to be mediated by a 258-bp fragment of the hTERT promoter, proximal to the ATG initiation codon. Previous studies have demonstrated that overexpression of Myc can activate hTERT expression, suggesting that Myc may be a mediator of E6-mediated hTERT induction. However, in cells stably expressing E6, no strict correlation between the level of Myc and the activation of hTERT was found. Consistent with this observation, mutation of the two Myc binding sites in the hTERT promoter only modestly reduced responsiveness to E6 in transient reporter assays. This indicates that activation of Myc-dependent transcription is not essential for E6-mediated upregulation of hTERT expression. The hTERT promoter also contains five GC-rich elements that can bind Sp1. Mutation of these sites within the 258-bp fragment partially reduced hTERT induction by E6. However, when mutations in the Sp1 sites were combined with the mutated Myc binding sites, all activation by E6 was lost. This indicates that it is the combinatorial binding of factors to Myc and Sp1 cis elements that is responsible for hTERT induction by E6.
Figures



References
-
- Bodnar A G, Ouellette M, Frolkis M, Holt S E, Chiu C P, Morin G B, Harley C B, Shay J W, Lichtsteiner S, Wright W E. Extension of life-span by introduction of telomerase into normal human cells. Science. 1998;279:349–352. - PubMed
-
- Cannistra S A, Niloff J M. Cancer of the uterine cervix. N Engl J Med. 1996;334:1030–1038. - PubMed
-
- Colgin L M, Reddel R R. Telomere maintenance mechanisms and cellular immortalization. Curr Opin Genet Dev. 1999;9:97–103. . (Erratum, 9:247.) - PubMed
-
- de Villiers E M. Human pathogenic papillomavirus types: an update. Curr Top Microbiol Immunol. 1994;186:1–12. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

