Epidermal growth factor-induced tumor cell invasion and metastasis initiated by dephosphorylation and downregulation of focal adhesion kinase
- PMID: 11359909
- PMCID: PMC87064
- DOI: 10.1128/MCB.21.12.4016-4031.2001
Epidermal growth factor-induced tumor cell invasion and metastasis initiated by dephosphorylation and downregulation of focal adhesion kinase
Abstract
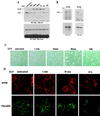
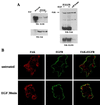
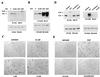
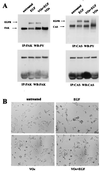
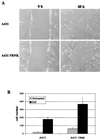
Upregulated epidermal growth factor (EGF) receptor (EGFR) expression and EGFR-induced signaling have been correlated with progression to invasion and metastasis in a wide variety of carcinomas, but the mechanism behind this is not well understood. We show here that, in various human carcinoma cells that overexpress EGFR, EGF treatment induced rapid tyrosine dephosphorylation of focal adhesion kinase (FAK) associated with downregulation of its kinase activity. The downregulation of FAK activity was both required and sufficient for EGF-induced refractile morphological changes, detachment of cells from the extracellular matrix, and increased tumor cell motility, invasion, and metastasis. Tumor cells with downregulated FAK activity became less adherent to the extracellular matrix. However, once cells started reattaching, FAK activity was restored by activated integrin signaling. Moreover, this process of readhesion and spreading could not be abrogated by further EGF stimulation. Interruption of transforming growth factor alpha-EGFR autocrine regulation with an EGFR tyrosine kinase inhibitor led to a substantial increase in FAK tyrosine phosphorylation and inhibition of tumor cell invasion in vitro. Consistent with this, FAK tyrosine phosphorylation was reduced in cells from tumors growing in transplanted, athymic, nude mice, which have an intact autocrine regulation of the EGFR. We suggest that the dynamic regulation of FAK activity, initiated by EGF-induced downregulation of FAK leading to cell detachment and increased motility and invasion, followed by integrin-dependent reactivation during readhesion, plays a role in EGF-associated tumor invasion and metastasis.
Figures







References
-
- Birchmeier W, Weidner K M, Behrens J. Molecular mechanisms leading to loss of differentiation and gain of invasiveness in epithelial cells. J Cell Sci Suppl. 1993;17:159–164. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
