UV-induced hyperphosphorylation of replication protein a depends on DNA replication and expression of ATM protein
- PMID: 11359916
- PMCID: PMC34578
- DOI: 10.1091/mbc.12.5.1199
UV-induced hyperphosphorylation of replication protein a depends on DNA replication and expression of ATM protein
Abstract
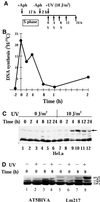
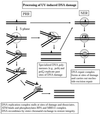
Exposure to DNA-damaging agents triggers signal transduction pathways that are thought to play a role in maintenance of genomic stability. A key protein in the cellular processes of nucleotide excision repair, DNA recombination, and DNA double-strand break repair is the single-stranded DNA binding protein, RPA. We showed previously that the p34 subunit of RPA becomes hyperphosphorylated as a delayed response (4-8 h) to UV radiation (10-30 J/m(2)). Here we show that UV-induced RPA-p34 hyperphosphorylation depends on expression of ATM, the product of the gene mutated in the human genetic disorder ataxia telangiectasia (A-T). UV-induced RPA-p34 hyperphosphorylation was not observed in A-T cells, but this response was restored by ATM expression. Furthermore, purified ATM kinase phosphorylates the p34 subunit of RPA complex in vitro at many of the same sites that are phosphorylated in vivo after UV radiation. Induction of this DNA damage response was also dependent on DNA replication; inhibition of DNA replication by aphidicolin prevented induction of RPA-p34 hyperphosphorylation by UV radiation. We postulate that this pathway is triggered by the accumulation of aberrant DNA replication intermediates, resulting from DNA replication fork blockage by UV photoproducts. Further, we suggest that RPA-p34 is hyperphosphorylated as a participant in the recombinational postreplication repair of these replication products. Successful resolution of these replication intermediates reduces the accumulation of chromosomal aberrations that would otherwise occur as a consequence of UV radiation.
Figures
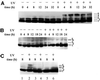
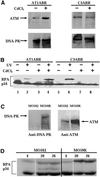
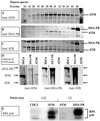


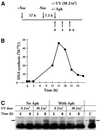
References
-
- Bender MA, Griggs HG, Bedford JS. Mechanisms of chromosomal aberration production. 3. Chemicals and ionizing radiation. Mutat Res. 1974;23:197–212. - PubMed
-
- Bender MA, Griggs HG, Walker PL. Mechanisms of chromosomal aberration production. I. Aberration induction by ultraviolet light. Mutat Res. 1973;20:387–402. - PubMed
-
- Bierne H, Michel B. When replication forks stop. Mol Microbiol. 1994;13:17–23. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

