Regulation of initiation of S phase, replication checkpoint signaling, and maintenance of mitotic chromosome structures during S phase by Hsk1 kinase in the fission yeast
- PMID: 11359920
- PMCID: PMC34582
- DOI: 10.1091/mbc.12.5.1257
Regulation of initiation of S phase, replication checkpoint signaling, and maintenance of mitotic chromosome structures during S phase by Hsk1 kinase in the fission yeast
Abstract
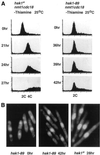
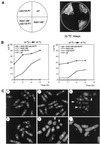
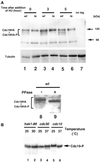
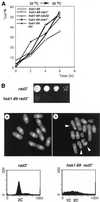
Hsk1, Saccharomyces cerevisiae Cdc7-related kinase in Shizosaccharomyces pombe, is required for G1/S transition and its kinase activity is controlled by the regulatory subunit Dfp1/Him1. Analyses of a newly isolated temperature-sensitive mutant, hsk1-89, reveal that Hsk1 plays crucial roles in DNA replication checkpoint signaling and maintenance of proper chromatin structures during mitotic S phase through regulating the functions of Rad3 (ATM)-Cds1 and Rad21 (cohesin), respectively, in addition to expected essential roles for initiation of mitotic DNA replication through phosphorylating Cdc19 (Mcm2). Checkpoint defect in hsk1-89 is indicated by accumulation of cut cells at 30 degrees C. hsk1-89 displays synthetic lethality in combination with rad3 deletion, indicating that survival of hsk1-89 depends on Rad3-dependent checkpoint pathway. Cds1 kinase activation, which normally occurs in response to early S phase arrest by nucleotide deprivation, is largely impaired in hsk1-89. Furthermore, Cds1-dependent hyperphosphorylation of Dfp1 in response to hydroxyurea arrest is eliminated in hsk1-89, suggesting that sufficient activation of Hsk1-Dfp1 kinase is required for S phase entry and replication checkpoint signaling. hsk1-89 displays apparent defect in mitosis at 37 degrees C leading to accumulation of cells with near 2C DNA content and with aberrant nuclear structures. These phenotypes are similar to those of rad21-K1 and are significantly enhanced in a hsk1-89 rad21-K1 double mutant. Consistent with essential roles of Rad21 as a component for the cohesin complex, sister chromatid cohesion is partially impaired in hsk1-89, suggesting a possibility that infrequent origin firing of the mutant may affect the cohesin functions during S phase.
Figures



Similar articles
-
A conserved domain of Schizosaccharomyces pombe dfp1(+) is uniquely required for chromosome stability following alkylation damage during S phase.Mol Cell Biol. 2002 Jul;22(13):4477-90. doi: 10.1128/MCB.22.13.4477-4490.2002. Mol Cell Biol. 2002. PMID: 12052858 Free PMC article.
-
A fission yeast gene, him1(+)/dfp1(+), encoding a regulatory subunit for Hsk1 kinase, plays essential roles in S-phase initiation as well as in S-phase checkpoint control and recovery from DNA damage.Mol Cell Biol. 1999 Aug;19(8):5535-47. doi: 10.1128/MCB.19.8.5535. Mol Cell Biol. 1999. PMID: 10409743 Free PMC article.
-
Hsk1-Dfp1/Him1, the Cdc7-Dbf4 kinase in Schizosaccharomyces pombe, associates with Swi1, a component of the replication fork protection complex.J Biol Chem. 2005 Dec 30;280(52):42536-42. doi: 10.1074/jbc.M510575200. Epub 2005 Oct 31. J Biol Chem. 2005. PMID: 16263721
-
Regulation of chromosome dynamics by Hsk1/Cdc7 kinase.Biochem Soc Trans. 2013 Dec;41(6):1712-9. doi: 10.1042/BST20130217. Biochem Soc Trans. 2013. PMID: 24256280 Review.
-
Cdc7 kinases (DDKs) and checkpoint responses: lessons from two yeasts.Mutat Res. 2003 Nov 27;532(1-2):21-7. doi: 10.1016/j.mrfmmm.2003.08.007. Mutat Res. 2003. PMID: 14643426 Review.
Cited by
-
Cdc7-Drf1 is a developmentally regulated protein kinase required for the initiation of vertebrate DNA replication.Genes Dev. 2005 Oct 1;19(19):2295-300. doi: 10.1101/gad.1339805. Genes Dev. 2005. PMID: 16204181 Free PMC article.
-
The origin recognition complex links replication, sister chromatid cohesion and transcriptional silencing in Saccharomyces cerevisiae.Genetics. 2004 Jun;167(2):579-91. doi: 10.1534/genetics.103.024851. Genetics. 2004. PMID: 15238513 Free PMC article.
-
Cds1 controls the release of Cdc14-like phosphatase Flp1 from the nucleolus to drive full activation of the checkpoint response to replication stress in fission yeast.Mol Biol Cell. 2008 Jun;19(6):2488-99. doi: 10.1091/mbc.e07-08-0737. Epub 2008 Apr 2. Mol Biol Cell. 2008. PMID: 18385517 Free PMC article.
-
A conserved domain of Schizosaccharomyces pombe dfp1(+) is uniquely required for chromosome stability following alkylation damage during S phase.Mol Cell Biol. 2002 Jul;22(13):4477-90. doi: 10.1128/MCB.22.13.4477-4490.2002. Mol Cell Biol. 2002. PMID: 12052858 Free PMC article.
-
RFCCtf18 and the Swi1-Swi3 complex function in separate and redundant pathways required for the stabilization of replication forks to facilitate sister chromatid cohesion in Schizosaccharomyces pombe.Mol Biol Cell. 2008 Feb;19(2):595-607. doi: 10.1091/mbc.e07-06-0618. Epub 2007 Nov 28. Mol Biol Cell. 2008. PMID: 18045993 Free PMC article.
References
-
- Aparicio OM, Weinstein DM, Bell SP. Components and dynamics of DNA replication complexes in S. cerevisiae: redistribution of MCM proteins and Cdc45 during S phase. Cell. 1997;91:59–69. - PubMed
-
- Bell S, Stillman B. Nucleotide-dependent recognition of chromosomal origins of DNA replication by a multiprotein complex. Nature. 1992;357:128–134. - PubMed
-
- Boddy MN, Furnari B, Mondesert O, Russell P. Replication checkpoint enforced by kinase Cds1 and Chk1. Science. 1998;280:909–912. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

