Role of Rab3 GDP/GTP exchange protein in synaptic vesicle trafficking at the mouse neuromuscular junction
- PMID: 11359932
- PMCID: PMC34594
- DOI: 10.1091/mbc.12.5.1421
Role of Rab3 GDP/GTP exchange protein in synaptic vesicle trafficking at the mouse neuromuscular junction
Abstract
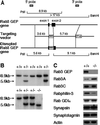
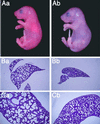
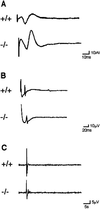
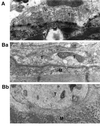
The Rab3 small G protein family consists of four members, Rab3A, -3B, -3C, and -3D. Of these members, Rab3A regulates Ca(2+)-dependent neurotransmitter release. These small G proteins are activated by Rab3 GDP/GTP exchange protein (Rab3 GEP). To determine the function of Rab3 GEP during neurotransmitter release, we have knocked out Rab3 GEP in mice. Rab3 GEP-/- mice developed normally but died immediately after birth. Embryos at E18.5 showed no evoked action potentials of the diaphragm and gastrocnemius muscles in response to electrical stimulation of the phrenic and sciatic nerves, respectively. In contrast, axonal conduction of the spinal cord and the phrenic nerve was not impaired. Total numbers of synaptic vesicles, especially those docked at the presynaptic plasma membrane, were reduced at the neuromuscular junction approximately 10-fold compared with controls, whereas postsynaptic structures and functions appeared normal. Thus, Rab3 GEP is essential for neurotransmitter release and probably for formation and trafficking of the synaptic vesicles.
Figures


Similar articles
-
A novel rabconnectin-3-binding protein that directly binds a GDP/GTP exchange protein for Rab3A small G protein implicated in Ca(2+)-dependent exocytosis of neurotransmitter.Genes Cells. 2003 Jun;8(6):537-46. doi: 10.1046/j.1365-2443.2003.00655.x. Genes Cells. 2003. PMID: 12786944
-
A GDP/GTP exchange protein for the Rab3 small G protein family up-regulates a postdocking step of synaptic exocytosis in central synapses.Proc Natl Acad Sci U S A. 2002 Oct 29;99(22):14536-41. doi: 10.1073/pnas.212511399. Epub 2002 Oct 18. Proc Natl Acad Sci U S A. 2002. PMID: 12388783 Free PMC article.
-
Rabconnectin-3, a novel protein that binds both GDP/GTP exchange protein and GTPase-activating protein for Rab3 small G protein family.J Biol Chem. 2002 Mar 22;277(12):9629-32. doi: 10.1074/jbc.C100730200. Epub 2002 Jan 24. J Biol Chem. 2002. PMID: 11809763
-
RAB3 and synaptotagmin: the yin and yang of synaptic membrane fusion.Annu Rev Neurosci. 1998;21:75-95. doi: 10.1146/annurev.neuro.21.1.75. Annu Rev Neurosci. 1998. PMID: 9530492 Review.
-
The synaptic vesicle cycle: a cascade of protein-protein interactions.Nature. 1995 Jun 22;375(6533):645-53. doi: 10.1038/375645a0. Nature. 1995. PMID: 7791897 Review.
Cited by
-
Accurate phenotypic classification and exome sequencing allow identification of novel genes and variants associated with adult-onset hearing loss.PLoS Genet. 2023 Nov 27;19(11):e1011058. doi: 10.1371/journal.pgen.1011058. eCollection 2023 Nov. PLoS Genet. 2023. PMID: 38011198 Free PMC article.
-
GTP binds to Rab3A in a complex with Ca2+/calmodulin.Biochem J. 2002 Mar 15;362(Pt 3):651-7. doi: 10.1042/0264-6021:3620651. Biochem J. 2002. PMID: 11879192 Free PMC article.
-
Distinct actions of Rab3 and Rab27 GTPases on late stages of exocytosis of insulin.Traffic. 2014 Sep;15(9):997-1015. doi: 10.1111/tra.12182. Epub 2014 Jun 26. Traffic. 2014. PMID: 24909540 Free PMC article.
-
Specific KIF1A-adaptor interactions control selective cargo recognition.J Cell Biol. 2021 Oct 4;220(10):e202105011. doi: 10.1083/jcb.202105011. Epub 2021 Jul 21. J Cell Biol. 2021. PMID: 34287616 Free PMC article.
-
A splice site variant in MADD affects hormone expression in pancreatic β cells and pituitary gonadotropes.JCI Insight. 2024 May 22;9(10):e167598. doi: 10.1172/jci.insight.167598. JCI Insight. 2024. PMID: 38775154 Free PMC article.
References
-
- Adachi R, Nigam R, Tuvim MJ, DeMayo F, Dickey BF. Genomic organization, chromosomal localization, and expression of the murine RAB3D gene. Biochem Biophys Res Commun. 2000;273:877–883. - PubMed
-
- Castillo PE, Janz R, Südhof TC, Tzounopoulos T, Malenka RC, Nicoll RA. Rab3A is essential for mossy fiber long-term potentiation in the hippocampus. Nature. 1997;388:590–593. - PubMed
-
- D'Adamo P, Menegon A, Lo Nigro C, Grasso M, Gulisano M, Tamanini F, Bienvenu T, Gedeon AK, Oostra B, Wu SK, Tandon A, Valtorta F, Balch WE, Chelly J, Toniolo D. Mutations in GDI1 are responsible for X-linked non-specific mental retardation. Nat Genet. 1998;19:134–139. - PubMed
-
- De Cammili P, Takei K. Molecular mechanisms in synaptic vesicle endocytosis and recycling. Neuron. 1996;16:481–486. - PubMed
-
- Dodd J, Morton SB, Karagogeos D, Yamamoto M, Jessell TM. Spatial regulation of axonal glycoprotein expression on subsets of embryonic spinal neurons. Neuron. 1988;1:105–116. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

