Induction of M3-restricted cytotoxic T lymphocyte responses by N-formylated peptides derived from Mycobacterium tuberculosis
- PMID: 11369792
- PMCID: PMC2193330
- DOI: 10.1084/jem.193.10.1213
Induction of M3-restricted cytotoxic T lymphocyte responses by N-formylated peptides derived from Mycobacterium tuberculosis
Abstract
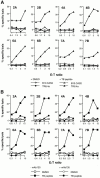
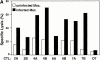
Major histocompatibility complex (MHC) class I-restricted CD8(+) T cells play a critical role in the protective immunity against Mycobacterium tuberculosis (Mtb). However, only a few Mtb peptides recognized by MHC class Ia-restricted CD8(+) T cells have been identified. Information on epitopes recognized by class Ib-restricted T cells is even more limited. M3 is an MHC class Ib molecule that preferentially presents N-formylated peptides to CD8(+) T cells. Because bacteria initiate protein synthesis with N-formyl methionine, the unique binding specificity of M3 makes it especially suitable for presenting these particular bacterial epitopes. We have scanned the full sequence of the Mtb genome for NH2-terminal peptides that share features with other M3-binding peptides. Synthetic peptides corresponding to these sequences were tested for their ability to bind to M3 in an immunofluorescence-based peptide-binding assay. Four of the N-formylated Mtb peptides were able to elicit cytotoxic T lymphocytes (CTLs) from mice immunized with peptide-coated splenocytes. The Mtb peptide-specific, M3-restricted CTLs lysed the Mtb-infected macrophages effectively, suggesting that these N-formylated Mtb peptides are presented as the naturally processed epitopes by Mtb-infected cells. Furthermore, T cells from Mtb-infected lungs, spleen, and lymph nodes responded to N-formylated Mtb peptides in an M3-restricted manner. Taken together, our data suggest that M3-restricted T cells may participate in the immune response to Mtb.
Figures
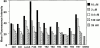



Comment in
-
CD8 T cell detection of bacterial infection: sniffing for formyl peptides derived from Mycobacterium tuberculosis.J Exp Med. 2001 May 21;193(10):F35-9. doi: 10.1084/jem.193.10.f35. J Exp Med. 2001. PMID: 11369794 Free PMC article. No abstract available.
References
-
- Enarson D.A., Murray J.F. Global epidemiology of tuberculosis. In: Rom W.M., Gary S.M., editors. Tuberculosis. Little, Brown and Company; New York: 1996. pp. 57–75.
-
- Canaday D.H., Ziebold C., Noss E.H., Chervenak K.A., Harding C.V., Boom W.H. Activation of human CD8+ αβ TCR+ cells by Mycobacterium tuberculosis via an alternate class I MHC antigen-processing pathway. J. Immunol. 1999;162:372–379. - PubMed
-
- Orme I.M., Collins F.M. Adoptive protection of the Mycobacterium tuberculosis-infected lung. Dissociation between cells that passively transfer protective immunity and those that transfer delayed-type hypersensitivity to tuberculin. Cell. Immunol. 1984;84:113–120. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

