Environmentally induced reversible conformational switching in the yeast cell adhesion protein alpha-agglutinin
- PMID: 11369849
- PMCID: PMC2374011
- DOI: 10.1110/ps.41701
Environmentally induced reversible conformational switching in the yeast cell adhesion protein alpha-agglutinin
Abstract
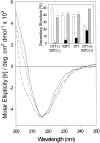
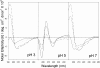
The yeast cell adhesion protein alpha-agglutinin is expressed on the surface of a free-living organism and is subjected to a variety of environmental conditions. Circular dichroism (CD) spectroscopy shows that the binding region of alpha-agglutinin has a beta-sheet-rich structure, with only approximately 2% alpha-helix under native conditions (15-40 degrees C at pH 5.5). This region is predicted to fold into three immunoglobulin-like domains, and models are consistent with the CD spectra as well as with peptide mapping and site-specific mutagenesis. However, secondary structure prediction algorithms show that segments comprising approximately 17% of the residues have high alpha-helical and low beta-sheet potential. Two model peptides of such segments had helical tendencies, and one of these peptides showed pH-dependent conformational switching. Similarly, CD spectroscopy of the binding region of alpha-agglutinin showed reversible conversion from beta-rich to mixed alpha/beta structure at elevated temperatures or when the pH was changed. The reversibility of these changes implied that there is a small energy difference between the all-beta and the alpha/beta states. Similar changes followed cleavage of peptide or disulfide bonds. Together, these observations imply that short sequences of high helical propensity are constrained to a beta-rich state by covalent and local charge interactions under native conditions, but form helices under non-native conditions.
Figures




References
-
- Baldwin, E.P., Hajiseyedjavadi, O., Baase, W.A., and Matthews, B.W. 1993. The role of backbone flexibility in the accommodation of variants that repack the core of T4 lysozyme. Science 262 1715–1718. - PubMed
-
- Baskakov, I.V., Aagaard, C., Mehlhorn, I., Wille, H., Groth, D., Baldwin, M.A., Prusiner, S.B., and Cohen, F.E. 2000. Self-assembly of recombinant prion protein of 106 residues. Biochemistry 39 2792–2804. - PubMed
-
- Bullough, P.A., Hughson, F.M., Skehel, J.J., and Wiley, D.C. 1994. Structure of influenza haemagglutinin at the pH of membrane fusion. Nature 371 37–43. - PubMed
-
- Carr, C.M. and Kim, P.S. 1993. A spring-loaded mechanism for the conformational change of influenza hemagglutinin. Cell 73 823–832. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

