Arabidopsis nph1 and npl1: blue light receptors that mediate both phototropism and chloroplast relocation
- PMID: 11371609
- PMCID: PMC34462
- DOI: 10.1073/pnas.101137598
Arabidopsis nph1 and npl1: blue light receptors that mediate both phototropism and chloroplast relocation
Abstract
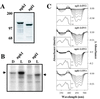
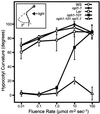
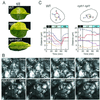
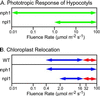
UV-A/blue light acts to regulate a number of physiological processes in higher plants. These include light-driven chloroplast movement and phototropism. The NPH1 gene of Arabidopsis encodes an autophosphorylating protein kinase that functions as a photoreceptor for phototropism in response to low-intensity blue light. However, nph1 mutants have been reported to exhibit normal phototropic curvature under high-intensity blue light, indicating the presence of an additional phototropic receptor. A likely candidate is the nph1 homologue, npl1, which has recently been shown to mediate the avoidance response of chloroplasts to high-intensity blue light in Arabidopsis. Here we demonstrate that npl1, like nph1, noncovalently binds the chromophore flavin mononucleotide (FMN) within two specialized PAS domains, termed LOV domains. Furthermore, when expressed in insect cells, npl1, like nph1, undergoes light-dependent autophosphorylation, indicating that npl1 also functions as a light receptor kinase. Consistent with this conclusion, we show that a nph1 npl1 double mutant exhibits an impaired phototropic response under both low- and high-intensity blue light. Hence, npl1 functions as a second phototropic receptor under high fluence rate conditions and is, in part, functionally redundant to nph1. We also demonstrate that both chloroplast accumulation in response to low-intensity light and chloroplast avoidance movement in response to high-intensity light are lacking in the nph1 npl1 double mutant. Our findings therefore indicate that nph1 and npl1 show partially overlapping functions in two different responses, phototropism and chloroplast relocation, in a fluence rate-dependent manner.
Figures
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

