Haploinsufficiency of Sox9 results in defective cartilage primordia and premature skeletal mineralization
- PMID: 11371614
- PMCID: PMC34415
- DOI: 10.1073/pnas.111092198
Haploinsufficiency of Sox9 results in defective cartilage primordia and premature skeletal mineralization
Abstract
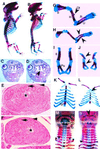
In humans, SOX9 heterozygous mutations cause the severe skeletal dysmorphology syndrome campomelic dysplasia. Except for clinical descriptions, little is known about the pathogenesis of this disease. We have generated heterozygous Sox9 mutant mice that phenocopy most of the skeletal abnormalities of this syndrome. The Sox9(+/-) mice died perinatally with cleft palate, as well as hypoplasia and bending of many skeletal structures derived from cartilage precursors. In embryonic day (E)14.5 heterozygous embryos, bending of radius, ulna, and tibia cartilages was already prominent. In E12.5 heterozygotes, all skeletal elements visualized by using Alcian blue were smaller. In addition, the overall levels of Col2a1 RNA at E10.5 and E12.5 were lower than in wild-type embryos. We propose that the skeletal abnormalities observed at later embryonic stages were caused by delayed or defective precartilaginous condensations. Furthermore, in E18.5 embryos and in newborn heterozygotes, premature mineralization occurred in many bones, including vertebrae and some craniofacial bones. Because Sox9 is not expressed in the mineralized portion of the growth plate, this premature mineralization is very likely the consequence of allele insufficiency existing in cells of the growth plate that express Sox9. Because the hypertrophic zone of the heterozygous Sox9 mutants was larger than that of wild-type mice, we propose that Sox9 also has a role in regulating the transition to hypertrophic chondrocytes in the growth plate. Despite the severe hypoplasia of cartilages, the overall organization and cellular composition of the growth plate were otherwise normal. Our results suggest the hypothesis that two critical steps of the chondrocyte differentiation pathway are sensitive to Sox9 dosage. First, an early step presumably at the stage of mesenchymal condensation of cartilage primordia, and second, a later step preceding the transition of chondrocytes into hypertrophic chondrocytes.
Figures
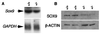




References
-
- Tacchetti C, Tracella S, Dozin B, Quarto R, Robino G, Cancedda R. Exp Cell Res. 1992;200:26–33. - PubMed
-
- Cancedda R, Descalzi Cancedda F, Castagnola P. Int Rev Cytol. 1995;159:265–358. - PubMed
-
- Bi W, Deng J M, Zhang Z, Behringer R R, de Crombrugghe B. Nat Genet. 1999;22:85–89. - PubMed
-
- Wright E, Hargrave M R, Christiansen J, Cooper L, Kun J, Evans T, Gangadharan U, Greenfield A, Koopman P. Nat Genet. 1995;9:15–20. - PubMed
-
- Zhao Q, Eberspaecher H, Lefebvre V, de Crombrugghe B. Dev Dyn. 1997;209:377–386. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

