Solution structure of DFF40 and DFF45 N-terminal domain complex and mutual chaperone activity of DFF40 and DFF45
- PMID: 11371636
- PMCID: PMC33420
- DOI: 10.1073/pnas.111145098
Solution structure of DFF40 and DFF45 N-terminal domain complex and mutual chaperone activity of DFF40 and DFF45
Abstract
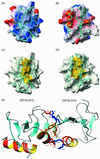
Apoptotic DNA fragmentation is mediated by a caspase-activated DNA fragmentation factor (DFF)40. Expression and folding of DFF40 require the presence of DFF45, which also acts as a nuclease inhibitor before DFF40 activation by execution caspases. The N-terminal domains (NTDs) of both proteins are homologous, and their interaction plays a key role in the proper functioning of this two-component system. Here we report that the NTD of DFF45 alone is unstructured in solution, and its folding is induced upon binding to DFF40 NTD. Therefore, folding of both proteins regulates the formation of the DFF40/DFF45 complex. The solution structure of the heterodimeric complex between NTDs of DFF40 and DFF45 reported here shows that the mutual chaperoning includes the formation of an extensive network of intermolecular interactions that bury a hydrophobic cluster inside the interface, surrounded by intermolecular salt bridges.
Figures


References
-
- Thompson C B. Science. 1995;267:1456–1462. - PubMed
-
- Enari M, Sakahira H, Yokoyama H, Okawa K, Iwamatsu A, Nagata S. Nature (London) 1998;391:43–50. - PubMed
-
- Sakahira H, Enari M, Nagata S. Nature (London) 1998;391:96–99. - PubMed
-
- Halenbeck R, MacDonald H, Roulston A, Chen T T, Conroy L, Williams L T. Curr Biol. 1998;8:537–540. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

