Osteoprotegerin inhibits prostate cancer-induced osteoclastogenesis and prevents prostate tumor growth in the bone
- PMID: 11375413
- PMCID: PMC209296
- DOI: 10.1172/JCI11685
Osteoprotegerin inhibits prostate cancer-induced osteoclastogenesis and prevents prostate tumor growth in the bone
Abstract
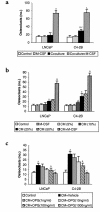
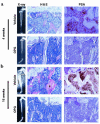
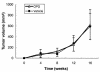
Prostate cancer (CaP) forms osteoblastic skeletal metastases with an underlying osteoclastic component. However, the importance of osteoclastogenesis in the development of CaP skeletal lesions is unknown. In the present study, we demonstrate that CaP cells directly induce osteoclastogenesis from osteoclast precursors in the absence of underlying stroma in vitro. CaP cells produced a soluble form of receptor activator of NF-kappaB ligand (RANKL), which accounted for the CaP-mediated osteoclastogenesis. To evaluate for the importance of osteoclastogenesis on CaP tumor development in vivo, CaP cells were injected both intratibially and subcutaneously in the same mice, followed by administration of the decoy receptor for RANKL, osteoprotegerin (OPG). OPG completely prevented the establishment of mixed osteolytic/osteoblastic tibial tumors, as were observed in vehicle-treated animals, but it had no effect on subcutaneous tumor growth. Consistent with the role of osteoclasts in tumor development, osteoclast numbers were elevated at the bone/tumor interface in the vehicle-treated mice compared with the normal values in the OPG-treated mice. Furthermore, OPG had no effect on CaP cell viability, proliferation, or basal apoptotic rate in vitro. These results emphasize the important role that osteoclast activity plays in the establishment of CaP skeletal metastases, including those with an osteoblastic component.
Figures



Comment in
-
Osteolysis and cancer.J Clin Invest. 2001 May;107(10):1219-20. doi: 10.1172/JCI13073. J Clin Invest. 2001. PMID: 11375409 Free PMC article. No abstract available.
References
-
- Abrams H, Spiro R, Goldstein N. Metastases in carcinoma. Cancer. 1950;3:74–85. - PubMed
-
- Charhon SA, et al. Histomorphometric analysis of sclerotic bone metastases from prostatic carcinoma special reference to osteomalacia. Cancer. 1983;51:918–924. - PubMed
-
- Urwin GH, et al. Generalised increase in bone resorption in carcinoma of the prostate. Br J Urol. 1985;57:721–723. - PubMed
-
- Clarke NW, McClure J, George NJ. Disodium pamidronate identifies differential osteoclastic bone resorption in metastatic prostate cancer. Br J Urol. 1992;69:64–70. - PubMed
-
- Clarke N. The effects of pamidronate disodium treatment in metastatic prostate cancer. Rev Contemp Pharamcother. 1998;9:205–212.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

