Homocysteine-induced endoplasmic reticulum stress causes dysregulation of the cholesterol and triglyceride biosynthetic pathways
- PMID: 11375416
- PMCID: PMC209295
- DOI: 10.1172/JCI11596
Homocysteine-induced endoplasmic reticulum stress causes dysregulation of the cholesterol and triglyceride biosynthetic pathways
Abstract
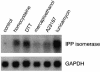
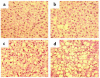
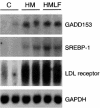
Hepatic steatosis is common in patients having severe hyperhomocysteinemia due to deficiency for cystathionine beta-synthase. However, the mechanism by which homocysteine promotes the development and progression of hepatic steatosis is unknown. We report here that homocysteine-induced endoplasmic reticulum (ER) stress activates both the unfolded protein response and the sterol regulatory element-binding proteins (SREBPs) in cultured human hepatocytes as well as vascular endothelial and aortic smooth muscle cells. Activation of the SREBPs is associated with increased expression of genes responsible for cholesterol/triglyceride biosynthesis and uptake and with intracellular accumulation of cholesterol. Homocysteine-induced gene expression was inhibited by overexpression of the ER chaperone, GRP78/BiP, thus demonstrating a direct role of ER stress in the activation of cholesterol/triglyceride biosynthesis. Consistent with these in vitro findings, cholesterol and triglycerides were significantly elevated in the livers, but not plasmas, of mice having diet-induced hyperhomocysteinemia. This effect was not due to impaired hepatic export of lipids because secretion of VLDL-triglyceride was increased in hyperhomocysteinemic mice. These findings suggest a mechanism by which homocysteine-induced ER stress causes dysregulation of the endogenous sterol response pathway, leading to increased hepatic biosynthesis and uptake of cholesterol and triglycerides. Furthermore, this mechanism likely explains the development and progression of hepatic steatosis and possibly atherosclerotic lesions observed in hyperhomocysteinemia.
Figures








Comment in
-
Hyperhomocysteinemia and function of the endoplasmic reticulum.J Clin Invest. 2001 May;107(10):1221-2. doi: 10.1172/JCI13092. J Clin Invest. 2001. PMID: 11375410 Free PMC article. No abstract available.
Similar articles
-
Unfolding new mechanisms of alcoholic liver disease in the endoplasmic reticulum.J Gastroenterol Hepatol. 2006 Oct;21 Suppl 3:S7-9. doi: 10.1111/j.1440-1746.2006.04581.x. J Gastroenterol Hepatol. 2006. PMID: 16958678 Review.
-
Predominant role of sterol response element binding proteins (SREBP) lipogenic pathways in hepatic steatosis in the murine intragastric ethanol feeding model.J Hepatol. 2006 Nov;45(5):717-24. doi: 10.1016/j.jhep.2006.05.009. Epub 2006 Jun 21. J Hepatol. 2006. PMID: 16879892
-
Homocysteine Induces Hepatic Steatosis Involving ER Stress Response in High Methionine Diet-Fed Mice.Nutrients. 2017 Apr 1;9(4):346. doi: 10.3390/nu9040346. Nutrients. 2017. PMID: 28368295 Free PMC article.
-
Hyperhomocysteinemia due to cystathionine beta synthase deficiency induces dysregulation of genes involved in hepatic lipid homeostasis in mice.J Hepatol. 2007 Jan;46(1):151-9. doi: 10.1016/j.jhep.2006.07.028. Epub 2006 Sep 22. J Hepatol. 2007. PMID: 17030070
-
Nutritional related liver disease: targeting the endoplasmic reticulum stress.Curr Opin Clin Nutr Metab Care. 2009 Nov;12(6):575-82. doi: 10.1097/MCO.0b013e32833189db. Curr Opin Clin Nutr Metab Care. 2009. PMID: 19726979 Review.
Cited by
-
Intracellular Ca2+ signaling and ORAI calcium release-activated calcium modulator 1 are associated with hepatic lipidosis in dairy cattle.J Anim Sci. 2021 Jul 1;99(7):skab184. doi: 10.1093/jas/skab184. J Anim Sci. 2021. PMID: 34100951 Free PMC article.
-
Cross-talk between lipid homeostasis and endoplasmic reticulum stress in neurodegeneration: Insights for HIV-1 associated neurocognitive disorders (HAND).Neurochem Int. 2020 Dec;141:104880. doi: 10.1016/j.neuint.2020.104880. Epub 2020 Oct 14. Neurochem Int. 2020. PMID: 33065212 Free PMC article. Review.
-
Interactions Between Nuclear Receptor SHP and FOXA1 Maintain Oscillatory Homocysteine Homeostasis in Mice.Gastroenterology. 2015 May;148(5):1012-1023.e14. doi: 10.1053/j.gastro.2015.01.045. Epub 2015 Feb 19. Gastroenterology. 2015. PMID: 25701738 Free PMC article.
-
Clinical Study of Serum Homocysteine and Non-Alcoholic Fatty Liver Disease in Euglycemic Patients.Med Sci Monit. 2016 Nov 2;22:4146-4151. doi: 10.12659/msm.897924. Med Sci Monit. 2016. PMID: 27803497 Free PMC article.
-
Role of Endoplasmic Reticulum Stress in Atherosclerosis and Its Potential as a Therapeutic Target.Oxid Med Cell Longev. 2020 Sep 9;2020:9270107. doi: 10.1155/2020/9270107. eCollection 2020. Oxid Med Cell Longev. 2020. PMID: 32963706 Free PMC article. Review.
References
-
- Ross R. The pathogenesis of atherosclerosis: a perspective for the 1990s. Nature. 1993;362:801–809. - PubMed
-
- McCully KS. Homocysteine and vascular disease. Nat Med. 1996;2:386–389. - PubMed
-
- Ueland PM, Refsum H. Plasma homocysteine, a risk factor for vascular disease: plasma levels in health, disease, and drug therapy. J Lab Clin Med. 1989;114:473–501. - PubMed
-
- Clarke R, et al. Hyperhomocysteinemia: an independent risk factor for vascular disease. N Engl J Med. 1991;324:1149–1155. - PubMed
-
- Selhub J, et al. Association between plasma homocysteine concentrations and extracranial carotid-artery stenosis. N Engl J Med. 1995;332:286–291. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

