Rescue of the early vascular defects in Tek/Tie2 null mice reveals an essential survival function
- PMID: 11375937
- PMCID: PMC1083887
- DOI: 10.1093/embo-reports/kve093
Rescue of the early vascular defects in Tek/Tie2 null mice reveals an essential survival function
Abstract
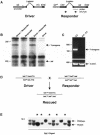
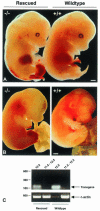
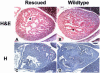
Disruption of the signaling pathways mediated by the receptor tyrosine kinase Tek/Tie2 has shown that this receptor plays a pivotal role in vascularization of the developing embryo. In this report, we have utilized the tetracycline-responsive binary transgenic system to overcome the early lethal cardiovascular defects associated with the tekDeltasp null allele in order to investigate the role of Tek in later stages of vessel growth. We show for the first time in vivo that synchronized loss of tek expression correlates with rapid endothelial cell apoptosis in hemorrhagic regions of the embryo, demonstrating an ongoing requirement for Tek-mediated signal transduction in vascular maintenance.
Figures



References
-
- Davis S. et al. (1996) Isolation of angiopoietin-1, a ligand for the TIE2 receptor, by secretion-trap expression cloning. Cell, 87, 1161–1169. - PubMed
-
- Dumont D.J., Yamaguchi, T.P., Conlon, R.A., Rossant, J. and Breitman, M.L. (1992) tek, a novel tyrosine kinase gene located on mouse chromosome 4, is expressed in endothelial cells and their presumptive precursors. Oncogene, 7, 1471–1480. - PubMed
-
- Dumont D.J., Gradwohl, G., Fong, G.H., Puri, M.C., Gertsenstein, M., Auerbach, A. and Breitman, M.L. (1994) Dominant-negative and targeted null mutations in the endothelial receptor tyrosine kinase, tek, reveal a critical role in vasculogenesis of the embryo. Genes Dev., 8, 1897–1909. - PubMed
-
- Dumont D.J., Fong, G.H., Puri, M.C., Gradwohl, G., Alitalo, K. and Breitman, M.L. (1995) Vascularization of the mouse embryo: a study of flk-1, tek, tie, and vascular endothelial growth factor expression during development. Dev. Dyn., 203, 80–92. - PubMed
-
- Folkman J. and D’Amore, P.A. (1996) Blood vessel formation: what is its molecular basis? Cell, 87, 1153–1155. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

