Insulin-like growth factor 1 and oestradiol promote cell proliferation of MCF-7 breast cancer cells: new insights into their synergistic effects
- PMID: 11376126
- PMCID: PMC1187053
- DOI: 10.1136/mp.54.3.149
Insulin-like growth factor 1 and oestradiol promote cell proliferation of MCF-7 breast cancer cells: new insights into their synergistic effects
Abstract
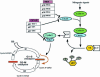
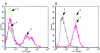
In MCF-7 breast cancer cells, the insulin-like growth factor 1 receptor (IGF-1R) and the oestrogen receptor (ER) are coexpressed and the two signalling systems are engaged in a crosstalk that results in synergistic growth. However, coupling between the signalling cascades is poorly understood. Oestradiol enhances IGF-1R signalling by inducing the expression of insulin receptor substrate 1 (IRS-1), a substrate of the IGF-1R. Oestradiol induced expression of IRS-1 results in enhanced tyrosine phosphorylation of IRS-1 after IGF-1 stimulation, followed by enhanced mitogen activated protein kinase, phosphoinositide 3' kinase, and Akt activation. Oestradiol can also potentiate the effect of IGF-1 on the expression of cyclin D1 and cyclin E, and on the phosphorylation of the retinoblastoma protein (RB). These effects are greatly diminished in SX13 cells, which exhibit a 50% reduction in IGF-1R expression but few functional IGF-1Rs at the surface. Oestradiol and IGF-1 regulate the expression of two cyclin dependent kinase inhibitors, p21 and p27, differently. Whereas IGF-1 increases p21 expression and reduces p27 expression, oestradiol has no effect on p21. In summary, in MCF-7 cells, oestrogen potentiates the effect of IGF-1 on IGF-1R signalling and its effects on certain cell cycle components.
Figures

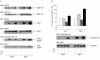
References
-
- Yee D. The insulin-like growth factor system as a target in breast cancer. Breast Cancer Res Treat 1994;32:85–95. - PubMed
-
- Pollack MW, Beamer W, Zhang JC. Insulin-like growth factors and prostate cancer. Cancer Metastasis Rev 1998;17:383–90. - PubMed
-
- Manousos OJ, Souglakos C, Bosetti A, et al. IGF-I and IGF-II in relation to colorectal cancer. Int J Cancer 1999;83:15–17. - PubMed
-
- Mishra LB, Bass BS, Ooi A, et al. Role of insulin-like growth factor-I (IGF-I) receptor, IGF-I, and IGF binding protein-2 in human colorectal cancers. Growth Horm IGF Res 1998;8:473–9. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
