Multiple features contribute to efficient constitutive splicing of an unusually large exon
- PMID: 11376148
- PMCID: PMC55698
- DOI: 10.1093/nar/29.11.2292
Multiple features contribute to efficient constitutive splicing of an unusually large exon
Abstract
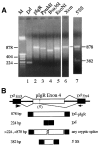
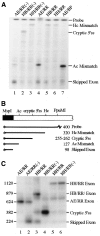
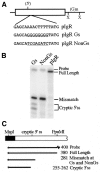
Vertebrate internal exons are usually between 50 and 400 nt long; exons outside this size range may require additional exonic and/or intronic sequences to be spliced into the mature mRNA. The mouse polymeric immunoglobulin receptor gene has a 654 nt exon that is efficiently spliced into the mRNA. We have examined this exon to identify features that contribute to its efficient splicing despite its large size; a large constitutive exon has not been studied previously. We found that a strong 5' splice site is necessary for this exon to be spliced intact, but the splice sites alone were not sufficient to efficiently splice a large exon. At least two exonic sequences and one evolutionarily conserved intronic sequence also contribute to recognition of this exon. However, these elements have redundant activities as they could only be detected in conjunction with other mutations that reduced splicing efficiency. Several mutations activated cryptic 5' splice sites that created smaller exons. Thus, the balance between use of these potential sites and the authentic 5' splice site must be modulated by sequences that repress or enhance use of these sites, respectively. Also, sequences that enhance cryptic splice site use must be absent from this large exon.
Figures







Similar articles
-
Cryptic intron activation within the large exon of the mouse polymeric immunoglobulin receptor gene: cryptic splice sites correspond to protein domain boundaries.Nucleic Acids Res. 1999 Sep 1;27(17):3446-54. doi: 10.1093/nar/27.17.3446. Nucleic Acids Res. 1999. PMID: 10446232 Free PMC article.
-
The "alternative" choice of constitutive exons throughout evolution.PLoS Genet. 2007 Nov;3(11):e203. doi: 10.1371/journal.pgen.0030203. PLoS Genet. 2007. PMID: 18020709 Free PMC article.
-
Characterization of disease-associated mutations affecting an exonic splicing enhancer and two cryptic splice sites in exon 13 of the cystic fibrosis transmembrane conductance regulator gene.Hum Mol Genet. 2003 Aug 15;12(16):2031-40. doi: 10.1093/hmg/ddg215. Hum Mol Genet. 2003. PMID: 12913074
-
Exonization of transposed elements: A challenge and opportunity for evolution.Biochimie. 2011 Nov;93(11):1928-34. doi: 10.1016/j.biochi.2011.07.014. Epub 2011 Jul 26. Biochimie. 2011. PMID: 21787833 Review.
-
Regulation of alternative RNA splicing by exon definition and exon sequences in viral and mammalian gene expression.J Biomed Sci. 2004 May-Jun;11(3):278-94. doi: 10.1007/BF02254432. J Biomed Sci. 2004. PMID: 15067211 Free PMC article. Review.
Cited by
-
Constructing Physical and Genomic Maps for Puccinia striiformis f. sp. tritici, the Wheat Stripe Rust Pathogen, by Comparing Its EST Sequences to the Genomic Sequence of P. graminis f. sp. tritici, the Wheat Stem Rust Pathogen.Comp Funct Genomics. 2009;2009:302620. doi: 10.1155/2009/302620. Epub 2010 Feb 11. Comp Funct Genomics. 2009. PMID: 20169145 Free PMC article.
-
Splicing at the phase-separated nuclear speckle interface: a model.Nucleic Acids Res. 2021 Jan 25;49(2):636-645. doi: 10.1093/nar/gkaa1209. Nucleic Acids Res. 2021. PMID: 33337476 Free PMC article. Review.
-
Regulated splicing of large exons is linked to phase-separation of vertebrate transcription factors.EMBO J. 2021 Nov 15;40(22):e107485. doi: 10.15252/embj.2020107485. Epub 2021 Oct 4. EMBO J. 2021. PMID: 34605568 Free PMC article.
-
Oriented scanning is the leading mechanism underlying 5' splice site selection in mammals.PLoS Genet. 2006 Sep 1;2(9):e138. doi: 10.1371/journal.pgen.0020138. Epub 2006 Jul 20. PLoS Genet. 2006. PMID: 16948532 Free PMC article.
-
B-cell and plasma-cell splicing differences: a potential role in regulated immunoglobulin RNA processing.RNA. 2003 Oct;9(10):1264-73. doi: 10.1261/rna.5820103. RNA. 2003. PMID: 13130140 Free PMC article.
References
-
- Berget S.M. (1995) Exon recognition in vertebrate splicing. J. Biol. Chem., 270, 2411–2414. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

