Rapid multiserotype detection of human rhinoviruses on optically coated silicon surfaces
- PMID: 11378491
- PMCID: PMC7128216
- DOI: 10.1016/s1386-6532(01)00150-0
Rapid multiserotype detection of human rhinoviruses on optically coated silicon surfaces
Abstract
Background: More than 100 immunologically distinct serotypes of human rhinoviruses (HRV) have been discovered, making detection of surface exposed capsid antigens impractical. However, the non-structural protein 3C protease (3Cpro) is essential for viral replication and is relatively highly conserved among serotypes, making it a potential target for diagnostic testing. The thin film biosensor is an assay platform that can be formatted into a sensitive immunoassay for viral proteins in clinical specimens. The technology utilizes an optically coated silicon surface to convert specific molecular binding events into visual color changes by altering the reflective properties of light through molecular thin films.
Objective: To develop a rapid test for detection of HRV by developing broadly serotype reactive antibodies to 3Cpro and utilizing them in the thin film biosensor format.
Study design: Polyclonal antibodies to 3Cpro were purified and incorporated into the thin film assay. The in vitro sensitivity, specificity and multiserotype cross-reactivity of the 3Cpro assay were tested. Nasal washes from naturally infected individuals were also tested to verify that 3Cpro was detectable in clinical specimens.
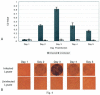
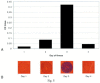
Results: The 3Cpro assay is a 28-min, non-instrumented room temperature test with a visual limit of detection of 12 pM (picomolar) 3Cpro. In terms of viral titer, as few as 1000 TCID(50) equivalents of HRV2 were detectable. The assay detected 45/52 (87%) of the HRV serotypes tested but showed no cross-reactivity to common respiratory viruses or bacteria. The thin film assay detected 3Cpro in HRV-infected cell culture supernatants coincident with first appearance of cytopathic effect. Data are also presented demonstrating 3Cpro detection from clinical samples collected from HRV-infected individuals. The assay detected 3Cpro in expelled nasal secretions from a symptomatic individual on the first day of illness. In addition, 9/11 (82%) concentrated nasal wash specimens from HRV infected children were positive in the 3Cpro test.
Conclusion: We have described a novel, sensitive thin film biosensor for rapid detection of HRV 3Cpro. This test may be suitable for the point of care setting, where rapid HRV diagnostic test results could contribute to clinical decisions regarding appropriate antibiotic or antiviral therapy.
Figures



References
-
- Arruda E., Boyle T.R., Winther B., Pevear D.C., Gwaltney J.M., Jr., Hayden F.G. Localization of human rhinovirus replication in the upper respiratory tract by in situ hybridization. J. Infect. Dis. 1995;171:1329–1333. - PubMed
-
- Calhoun A.M., Jordan W.S., Jr., Gwaltney J.M., Jr. Rhinovirus infections in an industrial population. Am. J. Epidemiol. 1974;99:58–64. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

