Neuropeptides and neuropeptide receptors in the Drosophila melanogaster genome
- PMID: 11381038
- PMCID: PMC311076
- DOI: 10.1101/gr.169901
Neuropeptides and neuropeptide receptors in the Drosophila melanogaster genome
Abstract
Recent genetic analyses in worms, flies, and mammals illustrate the importance of bioactive peptides in controlling numerous complex behaviors, such as feeding and circadian locomotion. To pursue a comprehensive genetic analysis of bioactive peptide signaling, we have scanned the recently completed Drosophila genome sequence for G protein-coupled receptors sensitive to bioactive peptides (peptide GPCRs). Here we describe 44 genes that represent the vast majority, and perhaps all, of the peptide GPCRs encoded in the fly genome. We also scanned for genes encoding potential ligands and describe 22 bioactive peptide precursors. At least 32 Drosophila peptide receptors appear to have evolved from common ancestors of 15 monophyletic vertebrate GPCR subgroups (e.g., the ancestral gastrin/cholecystokinin receptor). Six pairs of receptors are paralogs, representing recent gene duplications. Together, these findings shed light on the evolutionary history of peptide GPCRs, and they provide a template for physiological and genetic analyses of peptide signaling in Drosophila.
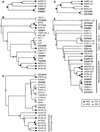
Figures


References
-
- Adams MD, Celniker SE, Holt RA, Evans CA, Gocayne JD, Amanatides PG, Scherer SE, Li PW, Hoskins RA, Galle RF, et al. The genome sequence of Drosophila melanogaster. Science. 2000;287:2185–2195. - PubMed
-
- Baines RA, Thompson KS, Rayne RC, Bacon JP. Analysis of the peptide content of the locust vasopressin-like immunoreactive (VPLI) neurons. Peptides. 1995;16:799–807. - PubMed
-
- Baldwin JM, Schertler GF, Unger VM. An α-carbon template for the transmembrane helices in the rhodopsin family of G-protein-coupled receptors. J Mol Biol. 1997;272:144–164. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
