Cytokinesis in prokaryotes and eukaryotes: common principles and different solutions
- PMID: 11381104
- PMCID: PMC99029
- DOI: 10.1128/MMBR.65.2.319-333.2001
Cytokinesis in prokaryotes and eukaryotes: common principles and different solutions
Abstract
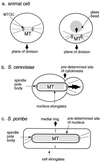
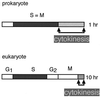
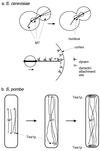
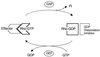
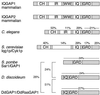
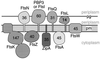
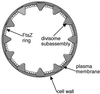
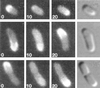
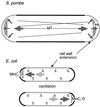
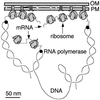
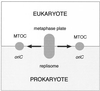
Cytokinesis requires duplication of cellular structures followed by bipolarization of the predivisional cell. As a common principle, this applies to prokaryotes as well as eukaryotes. With respect to eukaryotes, the discussion has focused mainly on Saccharomyces cerevisiae and on Schizosaccharomyces pombe. Escherichia coli and to a lesser extent Bacillus subtilis have been used as prokaryotic examples. To establish a bipolar cell, duplication of a eukaryotic origin of DNA replication as well as its genome is not sufficient. Duplication of the microtubule-organizing center is required as a prelude to mitosis, and it is here that the dynamic cytoskeleton with all its associated proteins comes to the fore. In prokaryotes, a cytoskeleton that pervades the cytoplasm appears to be absent. DNA replication and the concomitant DNA segregation seem to occur without help from extensive cytosolic supramacromolecular assemblies but with help from the elongating cellular envelope. Prokaryotic cytokinesis proceeds through a contracting ring, which has a roughly 100-fold-smaller circumference than its eukaryotic counterpart. Although the ring contains proteins that can be considered as predecessors of actin, tubulin, and microtubule-associated proteins, its macromolecular composition is essentially different.
Figures



Similar articles
-
[The origin of the eukaryotic cell. IV. The general hypothesis of the autogenous origin of eukaryotes].Tsitologiia. 1986 Sep;28(9):899-910. Tsitologiia. 1986. PMID: 3541331 Review. Russian.
-
Predation between prokaryotes and the origin of eukaryotes.Bioessays. 2009 Jul;31(7):748-57. doi: 10.1002/bies.200900018. Bioessays. 2009. PMID: 19492355 Review.
-
Gene co-regulation is highly conserved in the evolution of eukaryotes and prokaryotes.Nucleic Acids Res. 2004 Sep 7;32(16):4725-31. doi: 10.1093/nar/gkh815. Print 2004. Nucleic Acids Res. 2004. PMID: 15353560 Free PMC article.
-
Eukaryotic evolution, changes and challenges.Nature. 2006 Mar 30;440(7084):623-30. doi: 10.1038/nature04546. Nature. 2006. PMID: 16572163 Review.
-
The ring of life provides evidence for a genome fusion origin of eukaryotes.Nature. 2004 Sep 9;431(7005):152-5. doi: 10.1038/nature02848. Nature. 2004. PMID: 15356622
Cited by
-
Sublethal high hydrostatic pressure treatment reveals the importance of genes coding cytoskeletal protein in Escherichia coli morphogenesis.Curr Microbiol. 2013 Nov;67(5):515-21. doi: 10.1007/s00284-013-0392-8. Epub 2013 May 26. Curr Microbiol. 2013. PMID: 23708427
-
Biochemistry and comparative genomics of SxxK superfamily acyltransferases offer a clue to the mycobacterial paradox: presence of penicillin-susceptible target proteins versus lack of efficiency of penicillin as therapeutic agent.Microbiol Mol Biol Rev. 2002 Dec;66(4):702-38, table of contents. doi: 10.1128/MMBR.66.4.702-738.2002. Microbiol Mol Biol Rev. 2002. PMID: 12456788 Free PMC article. Review.
-
The zoonotic potential of daptomycin non-susceptible enterococci.Zoonoses Public Health. 2015 Feb;62(1):1-6. doi: 10.1111/zph.12091. Epub 2013 Nov 26. Zoonoses Public Health. 2015. PMID: 24274811 Free PMC article. Review.
-
Dictyostelium discoideum SecG interprets cAMP-mediated chemotactic signals to influence actin organization.Cytoskeleton (Hoboken). 2013 May;70(5):269-80. doi: 10.1002/cm.21107. Epub 2013 Apr 5. Cytoskeleton (Hoboken). 2013. PMID: 23564751 Free PMC article.
-
Prokaryotic DNA segregation by an actin-like filament.EMBO J. 2002 Jun 17;21(12):3119-27. doi: 10.1093/emboj/cdf320. EMBO J. 2002. PMID: 12065424 Free PMC article.
References
-
- Alberts B, Bray D, Lewis J, Raff M, Roberts K, Watson J D. Molecular biology of the cell. 3rd ed. New York and London: Garland Publishing, Inc.; 1994.
-
- Ball A R, Jr, Yokomori K. The structural maintenance of chromosomes (SMC) family of proteins in mammals. Chromosome Res. 2001;9:85–96. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

