Large conformational changes of the epsilon subunit in the bacterial F1F0 ATP synthase provide a ratchet action to regulate this rotary motor enzyme
- PMID: 11381110
- PMCID: PMC34392
- DOI: 10.1073/pnas.111128098
Large conformational changes of the epsilon subunit in the bacterial F1F0 ATP synthase provide a ratchet action to regulate this rotary motor enzyme
Abstract
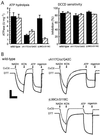
The F(1)F(0) ATP synthase is the smallest motor enzyme known. Previous studies had established that the central stalk, made of the gamma and epsilon subunits in the F(1) part and c subunit ring in the F(0) part, rotates relative to a stator composed of alpha(3)beta(3)deltaab(2) during ATP hydrolysis and synthesis. How this rotation is regulated has been less clear. Here, we show that the epsilon subunit plays a key role by acting as a switch of this motor. Two different arrangements of the epsilon subunit have been visualized recently. The first has been observed in beef heart mitochondrial F(1)-ATPase where the C-terminal portion is arranged as a two-alpha-helix hairpin structure that extends away from the alpha(3)beta(3) region, and toward the position of the c subunit ring in the intact F(1)F(0). The second arrangement was observed in a structure determination of a complex of the gamma and epsilon subunits of the Escherichia coli F(1)-ATPase. In this, the two C-terminal helices are apart and extend along the gamma to interact with the alpha and beta subunits in the intact complex. We have been able to trap these two arrangements by cross-linking after introducing appropriate Cys residues in E. coli F(1)F(0), confirming that both conformations of the epsilon subunit exist in the enzyme complex. With the C-terminal domain of epsilon toward the F(0), ATP hydrolysis is activated, but the enzyme is fully coupled in both ATP hydrolysis and synthesis. With the C-terminal domain toward the F(1) part, ATP hydrolysis is inhibited and yet the enzyme is fully functional in ATP synthesis; i.e., it works in one direction only. These results help explain the inhibitory action of the epsilon subunit in the F(1)F(0) complex and argue for a ratchet function of this subunit.
Figures


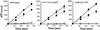
Similar articles
-
Rotor/Stator interactions of the epsilon subunit in Escherichia coli ATP synthase and implications for enzyme regulation.J Biol Chem. 2004 Aug 20;279(34):35616-21. doi: 10.1074/jbc.M405012200. Epub 2004 Jun 15. J Biol Chem. 2004. PMID: 15199054
-
Coupling H+ transport and ATP synthesis in F1F0-ATP synthases: glimpses of interacting parts in a dynamic molecular machine.J Exp Biol. 1997 Jan;200(Pt 2):217-24. doi: 10.1242/jeb.200.2.217. J Exp Biol. 1997. PMID: 9050229 Review.
-
Inhibition of ATP hydrolysis by thermoalkaliphilic F1Fo-ATP synthase is controlled by the C terminus of the epsilon subunit.J Bacteriol. 2006 Jun;188(11):3796-804. doi: 10.1128/JB.00040-06. J Bacteriol. 2006. PMID: 16707672 Free PMC article.
-
Structure of a thermophilic F1-ATPase inhibited by an ε-subunit: deeper insight into the ε-inhibition mechanism.FEBS J. 2015 Aug;282(15):2895-913. doi: 10.1111/febs.13329. Epub 2015 Jun 19. FEBS J. 2015. PMID: 26032434
-
Structural model of the transmembrane Fo rotary sector of H+-transporting ATP synthase derived by solution NMR and intersubunit cross-linking in situ.Biochim Biophys Acta. 2002 Oct 11;1565(2):232-45. doi: 10.1016/s0005-2736(02)00572-2. Biochim Biophys Acta. 2002. PMID: 12409198 Review.
Cited by
-
Catalytic robustness and torque generation of the F1-ATPase.Biophys Rev. 2017 Mar 25;9(2):103-118. doi: 10.1007/s12551-017-0262-x. eCollection 2017 Apr. Biophys Rev. 2017. PMID: 28424741 Free PMC article. Review.
-
The Inhibitory Mechanism of the ζ Subunit of the F1FO-ATPase Nanomotor of Paracoccus denitrificans and Related α-Proteobacteria.J Biol Chem. 2016 Jan 8;291(2):538-46. doi: 10.1074/jbc.M115.688143. Epub 2015 Nov 6. J Biol Chem. 2016. PMID: 26546676 Free PMC article.
-
A conformational change of the γ subunit indirectly regulates the activity of cyanobacterial F1-ATPase.J Biol Chem. 2012 Nov 9;287(46):38695-704. doi: 10.1074/jbc.M112.395053. Epub 2012 Sep 25. J Biol Chem. 2012. PMID: 23012354 Free PMC article.
-
Cross-linking of the endogenous inhibitor protein (IF1) with rotor (gamma, epsilon) and stator (alpha) subunits of the mitochondrial ATP synthase.J Bioenerg Biomembr. 2002 Dec;34(6):433-43. doi: 10.1023/a:1022514008462. J Bioenerg Biomembr. 2002. PMID: 12678435
-
The identification of Pcl1-interacting proteins that genetically interact with Cla4 may indicate a link between G1 progression and mitotic exit.Genetics. 2004 Mar;166(3):1177-86. doi: 10.1534/genetics.166.3.1177. Genetics. 2004. PMID: 15082539 Free PMC article.
References
-
- Senior A E. Annu Rev Biophys Biophys Chem. 1990;19:7–41. - PubMed
-
- Boyer P D. Annu Rev Biochem. 1997;66:717–749. - PubMed
-
- Capaldi R A, Schulenberg B, Murray J, Aggeler R. J Exp Biol. 2000;203:29–33. - PubMed
-
- Gogol E P, Aggeler R, Sagermann M, Capaldi R A. Biochemistry. 1989;28:4717–4724. - PubMed
-
- Abrahams J P, Leslie A G W, Lutter R, Walker J E. Nature (London) 1994;370:621–628. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

