Hyperphosphorylation induces self-assembly of tau into tangles of paired helical filaments/straight filaments
- PMID: 11381127
- PMCID: PMC34454
- DOI: 10.1073/pnas.121119298
Hyperphosphorylation induces self-assembly of tau into tangles of paired helical filaments/straight filaments
Abstract
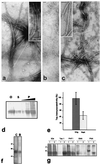
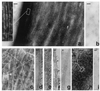
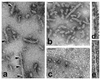
The microtubule-associated protein tau is a family of six isoforms that becomes abnormally hyperphosphorylated and accumulates in the form of paired helical filaments (PHF) in the brains of patients with Alzheimer's disease (AD) and patients with several other tauopathies. Here, we show that the abnormally hyperphosphorylated tau from AD brain cytosol (AD P-tau) self-aggregates into PHF-like structures on incubation at pH 6.9 under reducing conditions at 35 degrees C during 90 min. In vitro dephosphorylation, but not deglycosylation, of AD P-tau inhibits its self-association into PHF. Furthermore, hyperphosphorylation induces self-assembly of each of the six tau isoforms into tangles of PHF and straight filaments, and the microtubule binding domains/repeats region in the absence of the rest of the molecule can also self-assemble into PHF. Thus, it appears that tau self-assembles by association of the microtubule binding domains/repeats and that the abnormal hyperphosphorylation promotes the self-assembly of tau into tangles of PHF and straight filaments by neutralizing the inhibitory basic charges of the flanking regions.
Figures

References
-
- Finch C, Tanzi R E. Science. 1997;278:407–411. - PubMed
-
- Tomlinson B E, Blessed G, Roth M J. Neurol Sci. 1970;11:205–242. - PubMed
-
- Arigada P A, Growdon J H, Hedley-White E T, Hyman B T. Neurology. 1992;42:631–639. - PubMed
-
- Grundke-Iqbal I, Iqbal K, Quinlan M, Tung Y-C, Zaidi M S, Wisniewski H M. J Biol Chem. 1986;261:6084–6089. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

