Endothelial cell surface F1-F0 ATP synthase is active in ATP synthesis and is inhibited by angiostatin
- PMID: 11381144
- PMCID: PMC34409
- DOI: 10.1073/pnas.131067798
Endothelial cell surface F1-F0 ATP synthase is active in ATP synthesis and is inhibited by angiostatin
Abstract
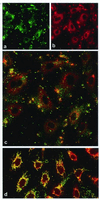
Angiostatin blocks tumor angiogenesis in vivo, almost certainly through its demonstrated ability to block endothelial cell migration and proliferation. Although the mechanism of angiostatin action remains unknown, identification of F(1)-F(O) ATP synthase as the major angiostatin-binding site on the endothelial cell surface suggests that ATP metabolism may play a role in the angiostatin response. Previous studies noting the presence of F(1) ATP synthase subunits on endothelial cells and certain cancer cells did not determine whether this enzyme was functional in ATP synthesis. We now demonstrate that all components of the F(1) ATP synthase catalytic core are present on the endothelial cell surface, where they colocalize into discrete punctate structures. The surface-associated enzyme is active in ATP synthesis as shown by dual-label TLC and bioluminescence assays. Both ATP synthase and ATPase activities of the enzyme are inhibited by angiostatin as well as by antibodies directed against the alpha- and beta-subunits of ATP synthase in cell-based and biochemical assays. Our data suggest that angiostatin inhibits vascularization by suppression of endothelial-surface ATP metabolism, which, in turn, may regulate vascular physiology by established mechanisms. We now have shown that antibodies directed against subunits of ATP synthase exhibit endothelial cell-inhibitory activities comparable to that of angiostatin, indicating that these antibodies function as angiostatin mimetics.
Figures


 ) (28).
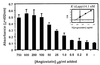
Angiostatin (10 μM) completely inhibited purified F1 ATP
synthase activity (◊), comparable to a known F1 ATP
synthase inhibitor, NaN3 (2%) (⧫) and an enzyme-free
control (▴). Polyclonal antibodies directed against the
recombinant α-subunit of ATP synthase (500 μg/ml) (▾)
and β-subunit ATP synthase (700 μg/ml) (▿) abolished
ATPase activity. A mAb to the α-subunit of ATP synthase (25
μg/ml) also inhibited activity (■). Control antibodies
had no effect on activity (○, □). Representative
data are shown; n = 3.
) (28).
Angiostatin (10 μM) completely inhibited purified F1 ATP
synthase activity (◊), comparable to a known F1 ATP
synthase inhibitor, NaN3 (2%) (⧫) and an enzyme-free
control (▴). Polyclonal antibodies directed against the
recombinant α-subunit of ATP synthase (500 μg/ml) (▾)
and β-subunit ATP synthase (700 μg/ml) (▿) abolished
ATPase activity. A mAb to the α-subunit of ATP synthase (25
μg/ml) also inhibited activity (■). Control antibodies
had no effect on activity (○, □). Representative
data are shown; n = 3.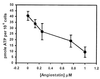
References
-
- Folkman J. In: Cancer Medicine. Holland J F, Frei E, Bast R, Kufe D, Morton D, Weichselbaum R, editors. Vol. 1. Baltimore: Williams & Wilkins; 1997. pp. 181–204.
-
- Gullino P M. J Natl Cancer Inst. 1978;61:639–643. - PubMed
-
- Hanahan D, Folkman J. Cell. 1996;86:353–364. - PubMed
-
- Folkman J, Watson K, Ingber D, Hanahan D. Nature (London) 1989;339:58–61. - PubMed
-
- Folkman J. N Engl J Med. 1971;285:1182–1186. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

