Integrated allosteric model of voltage gating of HCN channels
- PMID: 11382803
- PMCID: PMC2232403
- DOI: 10.1085/jgp.117.6.519
Integrated allosteric model of voltage gating of HCN channels
Abstract
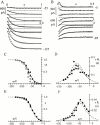
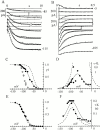
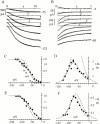
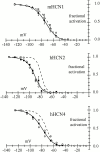
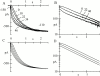
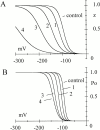
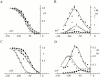
Hyperpolarization-activated (pacemaker) channels are dually gated by negative voltage and intracellular cAMP. Kinetics of native cardiac f-channels are not compatible with HH gating, and require closed/open multistate models. We verified that members of the HCN channel family (mHCN1, hHCN2, hHCN4) also have properties not complying with HH gating, such as sigmoidal activation and deactivation, activation deviating from fixed power of an exponential, removal of activation "delay" by preconditioning hyperpolarization. Previous work on native channels has indicated that the shifting action of cAMP on the open probability (Po) curve can be accounted for by an allosteric model, whereby cAMP binds more favorably to open than closed channels. We therefore asked whether not only cAMP-dependent, but also voltage-dependent gating of hyperpolarization-activated channels could be explained by an allosteric model. We hypothesized that HCN channels are tetramers and that each subunit comprises a voltage sensor moving between "reluctant" and "willing" states, whereas voltage sensors are independently gated by voltage, channel closed/open transitions occur allosterically. These hypotheses led to a multistate scheme comprising five open and five closed channel states. We estimated model rate constants by fitting first activation delay curves and single exponential time constant curves, and then individual activation/deactivation traces. By simply using different sets of rate constants, the model accounts for qualitative and quantitative aspects of voltage gating of all three HCN isoforms investigated, and allows an interpretation of the different kinetic properties of different isoforms. For example, faster kinetics of HCN1 relative to HCN2/HCN4 are attributable to higher HCN1 voltage sensors' rates and looser voltage-independent interactions between subunits in closed/open transitions. It also accounts for experimental evidence that reduction of sensors' positive charge leads to negative voltage shifts of Po curve, with little change of curve slope. HCN voltage gating thus involves two processes: voltage sensor gating and allosteric opening/closing.
Figures



References
-
- Chen J., Mitcheson J.S., Lin M., Sanguinetti M.C. Functional role of charged residues in the putative voltage sensor of the HCN2 pacemaker channel. J. Biol. Chem. 2000;275:36465–36471. - PubMed
-
- Clapham D.E. Not so funny anymorepacing channels are cloned. Neuron. 1998;21:1–5. - PubMed
-
- DiFrancesco D. The cardiac hyperpolarizing-activated current, if. Origins and developments. Prog. Biophys. Mol. Biol. 1985;46:163–183. - PubMed
-
- DiFrancesco D. Pacemaker mechanisms in cardiac tissue. Annu. Rev. Physiol. 1993;55:455–472. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

