Single-channel properties of recombinant acid-sensitive ion channels formed by the subunits ASIC2 and ASIC3 from dorsal root ganglion neurons expressed in Xenopus oocytes
- PMID: 11382806
- PMCID: PMC2232399
- DOI: 10.1085/jgp.117.6.563
Single-channel properties of recombinant acid-sensitive ion channels formed by the subunits ASIC2 and ASIC3 from dorsal root ganglion neurons expressed in Xenopus oocytes
Abstract
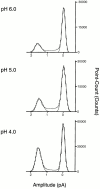
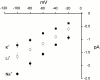
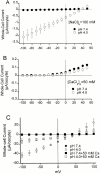
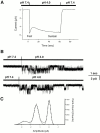
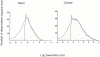
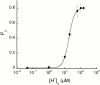
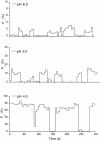
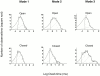
The acid-sensitive ion channels known as ASIC are gated by external protons. A set of these channels is expressed in dorsal root ganglion neurons where they may participate in the transduction of mechanical and nociceptive stimuli. Here, we have examined the single-channel properties of channels formed by the subunits ASIC2 and ASIC3 expressed in Xenopus oocytes using outside-out patches. The mean single-channel current-voltage relationship is linear with a slope conductance of 18 pS between -80 and -40 mV in 150 mM Na(+) outside and 150 mM K(+) inside the patch pipet. The selectivity for monovalent cations has the sequence Na(+) > Li(+) > K(+). Divalent cations such as Ca(2+) do not permeate, but instead block the channel when applied to the extracellular side. External protons increase the probability of channels being open to a maximum of 0.8 with an EC(50) of 16 +/- 4 microM and a Hill coefficient of 2.7 +/- 0.3, whereas the mean single-channel current amplitude is independent of external pH. Analysis of the kinetics of single channels indicates the presence of at least four modes of activity (Mod1 to Mod4) in addition to an inactivated state. Three of the modes exhibit distinct kinetics, and can be unambiguously identified on the basis of open probability (P(oMod1) = 0.5 +/- 0.05; P(oMod2) > 0.9 +/- 0.05; P(oMod3) < 0.1). Mode 4, which has a P(o) in the range of 0.5-0.8, may constitute a distinct mode or alternatively, it represents transitions between the other three modes of activity. Increasing [H(+)](o) increases the frequency of entering the modes with high P(o) (modes 1, 2, and 4) and the time the channel spends in the modes with high activity.
Figures



Similar articles
-
Functional implications of the localization and activity of acid-sensitive channels in rat peripheral nervous system.Proc Natl Acad Sci U S A. 2002 Feb 19;99(4):2326-31. doi: 10.1073/pnas.042688199. Epub 2002 Feb 12. Proc Natl Acad Sci U S A. 2002. PMID: 11842212 Free PMC article.
-
Acid-sensing ion channels ASIC2 and ASIC3 do not contribute to mechanically activated currents in mammalian sensory neurones.J Physiol. 2004 May 1;556(Pt 3):691-710. doi: 10.1113/jphysiol.2003.058693. Epub 2004 Feb 27. J Physiol. 2004. PMID: 14990679 Free PMC article.
-
Single channel properties of rat acid-sensitive ion channel-1alpha, -2a, and -3 expressed in Xenopus oocytes.J Gen Physiol. 2002 Oct;120(4):553-66. doi: 10.1085/jgp.20028574. J Gen Physiol. 2002. PMID: 12356856 Free PMC article.
-
[Voltage-gated Na+ channel in nociceptive afferents of DRG neurons].Sheng Li Ke Xue Jin Zhan. 1999 Jul;30(3):198-202. Sheng Li Ke Xue Jin Zhan. 1999. PMID: 12532779 Review. Chinese.
-
Peptides inhibitors of acid-sensing ion channels.Toxicon. 2007 Feb;49(2):271-84. doi: 10.1016/j.toxicon.2006.09.026. Epub 2006 Oct 4. Toxicon. 2007. PMID: 17113616 Review.
Cited by
-
Silencing acid-sensing ion channel 1a alters cone-mediated retinal function.J Neurosci. 2006 May 24;26(21):5800-9. doi: 10.1523/JNEUROSCI.0344-06.2006. J Neurosci. 2006. PMID: 16723538 Free PMC article.
-
Derivatives of 2-aminobenzimidazole potentiate ASIC open state with slow kinetics of activation and desensitization.Front Physiol. 2023 Jan 12;14:1018551. doi: 10.3389/fphys.2023.1018551. eCollection 2023. Front Physiol. 2023. PMID: 36711018 Free PMC article.
-
Functional implications of the localization and activity of acid-sensitive channels in rat peripheral nervous system.Proc Natl Acad Sci U S A. 2002 Feb 19;99(4):2326-31. doi: 10.1073/pnas.042688199. Epub 2002 Feb 12. Proc Natl Acad Sci U S A. 2002. PMID: 11842212 Free PMC article.
-
Reductions in external divalent cations evoke novel voltage-gated currents in sensory neurons.PLoS One. 2012;7(2):e31585. doi: 10.1371/journal.pone.0031585. Epub 2012 Feb 6. PLoS One. 2012. PMID: 22328938 Free PMC article.
-
Drosophila ppk19 encodes a proton-gated and mechanosensitive ion channel.Sci Rep. 2022 Nov 1;12(1):18346. doi: 10.1038/s41598-022-23236-3. Sci Rep. 2022. PMID: 36319833 Free PMC article.
References
-
- Akaike N., Krishtal O.A., Maruyama T. Proton-induced sodium current in frog isolated dorsal root ganglion cells. J. Neurophysiol. 1990;63:805–813. - PubMed
-
- Akopian A.N., Chen C.C., Ding Y., Cesare P., Wood J.N. A new member of the acid-sensing ion channel family. Neuroreport. 2000;11:2217–2222. - PubMed
-
- Babinski K., Catarsi S., Biagini G., Séguéla P. Mammalian ASIC2a and ASIC3 subunits co-assemble into heteromeric proton-gated channels sensitive to Gd3+ . J. Biol. Chem. 2000;275:28519–28525. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

