Role of aquaporin water channels in airway fluid transport, humidification, and surface liquid hydration
- PMID: 11382807
- PMCID: PMC2232398
- DOI: 10.1085/jgp.117.6.573
Role of aquaporin water channels in airway fluid transport, humidification, and surface liquid hydration
Abstract
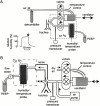
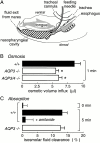
Several aquaporin-type water channels are expressed in mammalian airways and lung: AQP1 in microvascular endothelia, AQP3 in upper airway epithelia, AQP4 in upper and lower airway epithelia, and AQP5 in alveolar epithelia. Novel quantitative methods were developed to compare airway fluid transport-related functions in wild-type mice and knockout mice deficient in these aquaporins. Lower airway humidification, measured from the moisture content of expired air during mechanical ventilation with dry air through a tracheotomy, was 54-56% efficient in wild-type mice, and reduced by only 3-4% in AQP1/AQP5 or AQP3/AQP4 double knockout mice. Upper airway humidification, measured from the moisture gained by dry air passed through the upper airways in mice breathing through a tracheotomy, decreased from 91 to 50% with increasing ventilation from 20 to 220 ml/min, and reduced by 3-5% in AQP3/AQP4 knockout mice. The depth and salt concentration of the airway surface liquid in trachea was measured in vivo using fluorescent probes and confocal and ratio imaging microscopy. Airway surface liquid depth was 45 +/- 5 microm and [Na(+)] was 115 +/- 4 mM in wild-type mice, and not significantly different in AQP3/AQP4 knockout mice. Osmotic water permeability in upper airways, measured by an in vivo instillation/sample method, was reduced by approximately 40% by AQP3/AQP4 deletion. In doing these measurements, we discovered a novel amiloride-sensitive isosmolar fluid absorption process in upper airways (13% in 5 min) that was not affected by aquaporin deletion. These results establish the fluid transporting properties of mouse airways, and indicate that aquaporins play at most a minor role in airway humidification, ASL hydration, and isosmolar fluid absorption.
Figures



References
-
- Anderson S.D., Schoeffel R.E., Follet R., Perry C.P., Daviskas E., Kendall M. Sensitivity to heat and water loss at rest and during exercise in asthma patients. Eur. J. Respir. Dis. 1982;63:459–471. - PubMed
-
- Daviskas E., Gonda I., Anderson S.D. Mathematical modeling of heat and water transport in human respiratory tract. J. Appl. Physiol. 1990;69:362–372. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

