Identification of conserved C2H2 zinc-finger gene families in the Bilateria
- PMID: 11387037
- PMCID: PMC32188
- DOI: 10.1186/gb-2001-2-5-research0016
Identification of conserved C2H2 zinc-finger gene families in the Bilateria
Abstract
Background: Identification of orthologous relationships between genes from widely divergent taxa allows partial reconstruction of the gene complement of ancestral genomes. C2H2 zinc-finger genes are one of the largest and most complex gene superfamilies in metazoan genomes, with hundreds of members in the human genome. Here we analyze C2H2 zinc-finger genes from three taxa - Drosophila, Caenorhabditis elegans and human - from which near-complete genome sequence data are available.
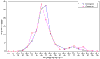
Results: Our analyses conclusively identify 39 families of genes, of which 38 can be defined as orthology groups in that they are descended from single ancestral genes in the common ancestor of Drosophila, C. elegans and humans.
Conclusions: On the basis of current metazoan phylogeny, these 39 groups represent the minimum complement of C2H2 zinc-finger genes present in the genome of the bilaterian common ancestor.
Figures
Similar articles
-
A survey of well conserved families of C2H2 zinc-finger genes in Daphnia.BMC Genomics. 2010 Apr 30;11:276. doi: 10.1186/1471-2164-11-276. BMC Genomics. 2010. PMID: 20433734 Free PMC article.
-
Comparative Genomics of the Zic Family Genes.Adv Exp Med Biol. 2018;1046:3-26. doi: 10.1007/978-981-10-7311-3_1. Adv Exp Med Biol. 2018. PMID: 29442314 Review.
-
C2H2 zinc finger genes of the Gli, Zic, KLF, SP, Wilms' tumour, Huckebein, Snail, Ovo, Spalt, Odd, Blimp-1, Fez and related gene families from Branchiostoma floridae.Dev Genes Evol. 2008 Dec;218(11-12):639-49. doi: 10.1007/s00427-008-0248-6. Epub 2008 Sep 16. Dev Genes Evol. 2008. PMID: 18795322
-
Comparative analysis of function and interaction of transcription factors in nematodes: extensive conservation of orthology coupled to rapid sequence evolution.BMC Genomics. 2008 Aug 27;9:399. doi: 10.1186/1471-2164-9-399. BMC Genomics. 2008. PMID: 18752680 Free PMC article.
-
Nuclear receptors in nematodes: themes and variations.Trends Genet. 2001 Apr;17(4):206-13. doi: 10.1016/s0168-9525(01)02242-9. Trends Genet. 2001. PMID: 11275326 Review.
Cited by
-
A study on the distribution of 37 well conserved families of C2H2 zinc finger genes in eukaryotes.BMC Genomics. 2013 Jun 24;14:420. doi: 10.1186/1471-2164-14-420. BMC Genomics. 2013. PMID: 23800006 Free PMC article.
-
High regulatory gene use in sea urchin embryogenesis: Implications for bilaterian development and evolution.Dev Biol. 2006 Dec 1;300(1):27-34. doi: 10.1016/j.ydbio.2006.10.016. Epub 2006 Oct 18. Dev Biol. 2006. PMID: 17101125 Free PMC article. Review.
-
Zinc-finger protein 545 inhibits cell proliferation as a tumor suppressor through inducing apoptosis and is disrupted by promoter methylation in breast cancer.PLoS One. 2014 Oct 31;9(10):e110990. doi: 10.1371/journal.pone.0110990. eCollection 2014. PLoS One. 2014. PMID: 25360542 Free PMC article.
-
Genomic AT Bias Coupled with Amino Acid Metabolism Modulates Codon Usage.J Mol Evol. 2025 Jun;93(3):379-394. doi: 10.1007/s00239-025-10251-x. Epub 2025 May 20. J Mol Evol. 2025. PMID: 40392286
-
Phylogenetic analysis of the human basic helix-loop-helix proteins.Genome Biol. 2002;3(6):RESEARCH0030. doi: 10.1186/gb-2002-3-6-research0030. Epub 2002 May 30. Genome Biol. 2002. PMID: 12093377 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

