C26-CoA-dependent ceramide synthesis of Saccharomyces cerevisiae is operated by Lag1p and Lac1p
- PMID: 11387200
- PMCID: PMC125493
- DOI: 10.1093/emboj/20.11.2655
C26-CoA-dependent ceramide synthesis of Saccharomyces cerevisiae is operated by Lag1p and Lac1p
Abstract
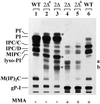
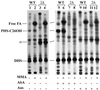
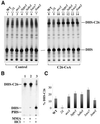
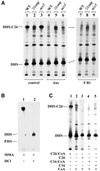
Lag1p and Lac1p are two highly homologous membrane proteins of the endoplasmic reticulum (ER). When both genes are deleted, cells cannot transport glycosylphosphatidylinositol (GPI)-anchored proteins from the ER to the Golgi at a normal rate. Here we show that microsomes or detergent extracts from lag1lac1 double mutants lack an activity transferring C26 fatty acids from C26-coenzyme A onto dihydrosphingosine or phytosphingosine. As a consequence, in intact cells, the normal ceramides and inositolphosphorylceramides are drastically reduced. lag1lac1 cells compensate for the lack of normal sphingolipids by making increased amounts of C26 fatty acids, which become incorporated into glycerophospholipids. They also contain 20- to 25-fold more free long chain bases than wild type and accumulate very large amounts of abnormally polar ceramides. They make small amounts of abnormal mild base-resistant inositolphospholipids. The lipid remodelling of GPI-anchored proteins is severely compromised in lag1lac1 double mutants since only few and mostly abnormal ceramides are incorporated into the GPI anchors. The participation of Lag1p and Lac1p in ceramide synthesis may explain their role in determining longevity.
Figures



References
-
- Beeler T.J., Fu,D., Rivera,J., Monaghan,E., Gable,K. and Dunn, T.M. (1997) SUR1 (CSG1/BCL21), a gene necessary for growth of Saccharomyces cerevisiae in the presence of high Ca2+ concentrations at 37°C, is required for mannosylation of inositolphosphorylceramide. Mol. Gen. Genet., 255, 570–579. - PubMed
-
- Beeler T., Bacikova,D., Gable,K., Hopkins,L., Johnson,C., Slife,H. and Dunn,T. (1998) The Saccharomyces cerevisiae TSC10/YBR265w gene encoding 3-ketosphinganine reductase is identified in a screen for temperature-sensitive suppressors of the Ca2+-sensitive csg2Δ mutant. J. Biol. Chem., 273, 30688–30694. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

