Novel voltage-dependent non-selective cation conductance in murine colonic myocytes
- PMID: 11389196
- PMCID: PMC2278626
- DOI: 10.1111/j.1469-7793.2001.0341a.x
Novel voltage-dependent non-selective cation conductance in murine colonic myocytes
Abstract
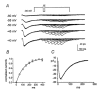
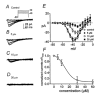
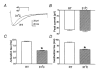
1. Two components of voltage-gated, inward currents were observed from murine colonic myocytes. One component had properties of L-type Ca(2+) currents and was inhibited by nicardipine (5 x 10(-7) M). A second component did not 'run down' during dialysis and was resistant to nicardipine (up to 10(-6) M). The nicardipine-insensitive current was activated by small depolarizations above the holding potential and reversed near 0 mV. 2. This low-voltage-activated current (I(LVA)) was resolved with step depolarizations positive to -60 mV, and the current rapidly inactivated upon sustained depolarization. The voltage of half-inactivation was -65 mV. Inactivation and activation time constants at -45 mV were 86 and 15 ms, respectively. The half-recovery time from inactivation was 98 ms at -45 mV. I(LVA) peaked at -40 mV and the current reversed at 0 mV. 3. I(LVA) was inhibited by Ni(2+) (IC(50) = 1.4 x 10(-5) M), mibefradil (10(-6) to 10(-5) M), and extracellular Ba(2+). Replacement of extracellular Na(+) with N-methyl-D-glucamine inhibited I(LVA) and shifted the reversal potential to -7 mV. Increasing extracellular Ca(2+) (5 x 10(-3) M) increased the amplitude of I(LVA) and shifted the reversal potential to +22 mV. I(LVA) was also blocked by extracellular Cs(+) (10(-4) M) and Gd(3+) (10(-6) M). 4. Warming increased the rates of activation and deactivation without affecting the amplitude of the peak current. 5. We conclude that the second component of voltage-dependent inward current in murine colonic myocytes is not a 'T-type' Ca(2+) current but rather a novel, voltage-gated non-selective cation current. Activation of this current could be important in the recovery of membrane potential following inhibitory junction potentials in gastrointestinal smooth muscle or in mediating responses to agonists.
Figures









Similar articles
-
Voltage-dependent inward currents of interstitial cells of Cajal from murine colon and small intestine.J Physiol. 2002 Jun 15;541(Pt 3):797-810. doi: 10.1113/jphysiol.2002.018796. J Physiol. 2002. PMID: 12068041 Free PMC article.
-
Inactivation properties of human recombinant class E calcium channels.J Neurophysiol. 2000 Feb;83(2):671-84. doi: 10.1152/jn.2000.83.2.671. J Neurophysiol. 2000. PMID: 10669483
-
LVA and HVA Ca(2+) currents in ventricular muscle cells of the Lymnaea heart.J Neurophysiol. 1999 Nov;82(5):2428-40. doi: 10.1152/jn.1999.82.5.2428. J Neurophysiol. 1999. PMID: 10561416
-
Voltage-dependent calcium channels.Gen Physiol Biophys. 2005 Jun;24 Suppl 1:1-78. Gen Physiol Biophys. 2005. PMID: 16096350 Review.
-
Voltage-dependent calcium channels in smooth muscle cells.J Cardiovasc Pharmacol. 1988;12 Suppl 6:S3-7. doi: 10.1097/00005344-198812006-00003. J Cardiovasc Pharmacol. 1988. PMID: 2468902 Review.
Cited by
-
Substance P modulates localized calcium transients and membrane current responses in murine colonic myocytes.Br J Pharmacol. 2003 Apr;138(7):1233-43. doi: 10.1038/sj.bjp.0705139. Br J Pharmacol. 2003. PMID: 12711623 Free PMC article.
-
T-type, but not L-type, voltage-gated calcium channels are dispensable for lymphatic pacemaking and spontaneous contractions.Sci Rep. 2020 Jan 9;10(1):70. doi: 10.1038/s41598-019-56953-3. Sci Rep. 2020. PMID: 31919478 Free PMC article.
-
Gastrointestinal motility and its enteric actors in mechanosensitivity: past and present.Pflugers Arch. 2015 Jan;467(1):191-200. doi: 10.1007/s00424-014-1635-7. Epub 2014 Nov 5. Pflugers Arch. 2015. PMID: 25366494 Review.
-
Role of Ca2+ entry and Ca2+ stores in atypical smooth muscle cell autorhythmicity in the mouse renal pelvis.Br J Pharmacol. 2007 Dec;152(8):1248-59. doi: 10.1038/sj.bjp.0707535. Epub 2007 Oct 29. Br J Pharmacol. 2007. PMID: 17965738 Free PMC article.
-
T- and L-type Ca2+ currents in freshly dispersed smooth muscle cells from the human proximal urethra.J Physiol. 2003 Aug 1;550(Pt 3):753-64. doi: 10.1113/jphysiol.2003.043695. Epub 2003 Jun 13. J Physiol. 2003. PMID: 12807987 Free PMC article.
References
-
- Bezprozvanny I, Tsien RW. Molecular Pharmacology. Vol. 48. 1995. Voltage-dependent blockade of diverse types of voltage-gated Ca2+ channels expressed in Xenopus oocytes by the Ca2+ channel antagonist mibefradil (Ro 40-5967) pp. 540–549. - PubMed
-
- Crist JR, He XD, Goyal RK. Chloride-mediated inhibitory junction potentials in opossum esophageal circular smooth muscle. American Journal of Physiology. 1991;261:G752–762. - PubMed
-
- Ertel SI, Ertel EA. Low-voltage-activated T-type Ca2+ channels. Trends in Pharmacological Sciences. 1997;18:37–42. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

