Excitation-contraction coupling in skeletal muscle of a mouse lacking the dihydropyridine receptor subunit gamma1
- PMID: 11389198
- PMCID: PMC2278637
- DOI: 10.1111/j.1469-7793.2001.0367a.x
Excitation-contraction coupling in skeletal muscle of a mouse lacking the dihydropyridine receptor subunit gamma1
Abstract
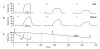
1. In skeletal muscle, dihydropyridine (DHP) receptors control both Ca(2+) entry (L-type current) and internal Ca(2+) release in a voltage-dependent manner. Here we investigated the question of whether elimination of the skeletal muscle-specific DHP receptor subunit gamma1 affects excitation-contraction (E-C) coupling. We studied intracellular Ca(2+) release and force production in muscle preparations of a mouse deficient in the gamma1 subunit (gamma-/-). 2. The rate of internal Ca(2+) release at large depolarization (+20 mV) was determined in voltage-clamped primary-cultured myotubes derived from satellite cells of adult mice by analysing fura-2 fluorescence signals and estimating the concentration of free and bound Ca(2+). On average, gamma-/- cells showed an increase in release of about one-third of the control value and no alterations in the time course. 3. Voltage of half-maximal activation (V(1/2)) and voltage sensitivity (k) were not significantly different in gamma-/- myotubes, either for internal Ca(2+) release activation or for the simultaneously measured L-type Ca(2+) conductance. The same was true for maximal Ca(2+) inward current and conductance. 4. Contractions evoked by electrical stimuli were recorded in isolated extensor digitorum longus (EDL; fast, glycolytic) and soleus (slow, oxidative) muscles under normal conditions and during fatigue induced by repetitive tetanic stimulation. Neither time course nor amplitudes of twitches and tetani nor force-frequency relations showed significant alterations in the gamma1-deficient muscles. 5. In conclusion, the overall results show that the gamma1 subunit is not essential for voltage-controlled Ca(2+) release and force production.
Figures
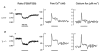


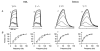
References
-
- Beam KG, Knudson CM, Powell JA. A lethal mutation in mice eliminates the slow calcium current in skeletal muscle cells. Nature. 1986;320:168–170. - PubMed
-
- Bosse E, Regulla S, Biel M, Ruth P, Meyer HE, Flockerzi V, Hofmann F. The cDNA and deduced amino acid sequence of the gamma subunit of the L-type calcium channel from rabbit skeletal muscle. FEBS Letters. 1990;267:153–156. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

