COX in a crystal ball: current status and future promise of prostaglandin research
- PMID: 11390412
- PMCID: PMC209326
- DOI: 10.1172/JCI13037
COX in a crystal ball: current status and future promise of prostaglandin research
Figures

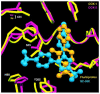
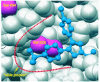
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

