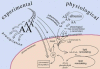
Arachidonic acid as a bioactive molecule
- PMID: 11390413
- PMCID: PMC209328
- DOI: 10.1172/JCI13210
Arachidonic acid as a bioactive molecule
Figures
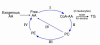
References
-
- Bild GS, Ramadoss CS, Axelrod B. Effect of solvent polarity on the activity of soybean lipoxygenase isozymes. Lipids. 1977;12:732–735.
-
- Glickman MH, Klinman JP. Nature of rate-limiting steps in the soybean lipoxygenase-1 reaction. Biochemistry. 1995;34:14077–14092. - PubMed
-
- Spector AA. Structure and lipid binding properties of serum albumin. J Lipid Res. 1986;128:320–329. - PubMed
-
- McArthur MJ, et al. Cellular uptake and intracellular trafficking of long chain fatty acids. J Lipid Res. 1999;40:1371–1383. - PubMed
-
- Berk PD, Stump DD. Mechanisms of cellular uptake of long chain free fatty acids. Mol Cell Biochem. 1999;192:17–31. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

