TNF-alpha downregulates murine hepatic growth hormone receptor expression by inhibiting Sp1 and Sp3 binding
- PMID: 11390427
- PMCID: PMC209317
- DOI: 10.1172/JCI10994
TNF-alpha downregulates murine hepatic growth hormone receptor expression by inhibiting Sp1 and Sp3 binding
Abstract
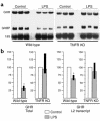
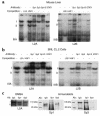
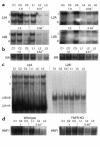
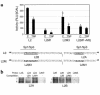
Children with chronic inflammatory diseases experience growth failure and wasting. This may be due to growth hormone resistance caused by cytokine-induced suppression of growth hormone receptor (GHR) gene expression. However, the factors governing inflammatory regulation of GHR are not known. We have reported that Sp1 and Sp3 regulate hepatic GHR expression. We hypothesized that TNF-alpha suppresses GHR expression by inhibiting Sp1/Sp3 transactivators. LPS administration significantly reduced murine hepatic GHR expression, as well as Sp1 and Sp3 binding to GHR promoter cis elements. TNF-alpha was integral to this response, as LPS did not affect hepatic Sp1/Sp3 binding or GHR expression in TNF receptor 1-deficient mice. TNF-alpha treatment of BNL CL.2 mouse liver cells reduced Sp1 and Sp3 binding to a GHR promoter cis element and downregulated activity of a GHR promoter-driven luciferase reporter. Combined mutations within adjacent Sp elements eliminated GHR promoter suppression by TNF-alpha without affecting overall nuclear levels of Sp1 or Sp3 proteins. These studies demonstrate that murine GHR transcription is downregulated by LPS, primarily via TNF-alpha-dependent signaling. Evidence suggests that inhibition of Sp transactivator binding is involved. Further investigation of these mechanisms may identify novel strategies for preventing inflammatory suppression of growth.
Figures
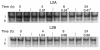

References
-
- Motil KJ, Grand RJ, Davis-Kraft L, Ferlic LL, Smith EO. Growth failure in children with inflammatory bowel disease: a prospective study. Gastroenterology. 1993;105:681–691. - PubMed
-
- Holt RI, et al. Hepatic growth hormone receptor, insulin-like growth factor I, and insulin-like growth factor-binding protein messenger RNA expression in pediatric liver disease. Hepatology. 1997;26:1600–1606. - PubMed
-
- Rosenbloom AL. Growth hormone insensitivity: physiologic and genetic basis, phenotype, and treatment. J Pediatr. 1999;135:280–289. - PubMed
-
- Beattie RM, et al. Responsiveness of IGF-I and IGFBP-3 to therapeutic intervention in children and adolescents with Crohn’s disease. Clin Endocrinol (Oxf) 1998;49:483–489. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

