Treatment of allergic airway inflammation and hyperresponsiveness by antisense-induced local blockade of GATA-3 expression
- PMID: 11390432
- PMCID: PMC2193377
- DOI: 10.1084/jem.193.11.1247
Treatment of allergic airway inflammation and hyperresponsiveness by antisense-induced local blockade of GATA-3 expression
Abstract
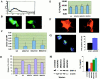
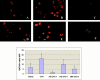
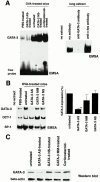
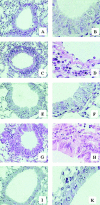
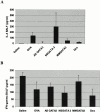
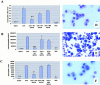
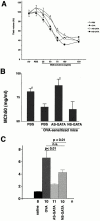
Recent studies in transgenic mice have revealed that expression of a dominant negative form of the transcription factor GATA-3 in T cells can prevent T helper cell type 2 (Th2)-mediated allergic airway inflammation in mice. However, it remains unclear whether GATA-3 plays a role in the effector phase of allergic airway inflammation and whether antagonizing the expression and/or function of GATA-3 can be used for the therapy of allergic airway inflammation and hyperresponsiveness. Here, we analyzed the effects of locally antagonizing GATA-3 function in a murine model of asthma. We could suppress GATA-3 expression in interleukin (IL)-4-producing T cells in vitro and in vivo by an antisense phosphorothioate oligonucleotide overlapping the translation start site of GATA-3, whereas nonsense control oligonucleotides were virtually inactive. In a murine model of asthma associated with allergic pulmonary inflammation and hyperresponsiveness in ovalbumin (OVA)-sensitized mice, local intranasal administration of fluorescein isothiocyanate-labeled GATA-3 antisense oligonucleotides led to DNA uptake in lung cells associated with a reduction of intracellular GATA-3 expression. Such intrapulmonary blockade of GATA-3 expression caused an abrogation of signs of lung inflammation including infiltration of eosinophils and Th2 cytokine production. Furthermore, treatment with antisense but not nonsense oligonucleotides induced a significant reduction of airway hyperresponsiveness in OVA-sensitized mice to levels comparable to saline-treated control mice, as assessed by both enhanced pause (PenH) responses and pulmonary resistance determined by body plethysmography. These data indicate a critical role for GATA-3 in the effector phase of a murine asthma model and suggest that local delivery of GATA-3 antisense oligonucleotides may be a novel approach for the treatment of airway hyperresponsiveness such as in asthma. This approach has the potential advantage of suppressing the expression of various proinflammatory Th2 cytokines simultaneously rather than suppressing the activity of a single cytokine.
Figures



References
-
- Kon O.M., Kay A.B. T cells and chronic asthma. Int. Arch. Allergy Immunol. 1999;118:133–135. - PubMed
-
- Kaliner M.A. Pathogenesis of asthma. In: Rich R.R., editor. Clinical Immunology. Mosby; St. Louis: 1996. pp. 909–923.
-
- Gelfand E.W. Essential role of T lymphocytes in the development of allergen-driven airway hyperresponsiveness. Allergy Asthma Proc. 1998;19:365–369. - PubMed
-
- Holgate S.T. The epidemic of allergy and asthma Nature. 402Suppl.1999. B2 B4 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

