Epstein-Barr virus immediate-early protein BRLF1 induces the lytic form of viral replication through a mechanism involving phosphatidylinositol-3 kinase activation
- PMID: 11390615
- PMCID: PMC114329
- DOI: 10.1128/JVI.75.13.6135-6142.2001
Epstein-Barr virus immediate-early protein BRLF1 induces the lytic form of viral replication through a mechanism involving phosphatidylinositol-3 kinase activation
Abstract
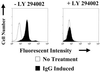
Expression of the Epstein-Barr virus (EBV) immediate-early (IE) protein BRLF1 induces the lytic form of viral replication in most EBV-positive cell lines. BRLF1 is a transcriptional activator that binds directly to a GC-rich motif present in some EBV lytic gene promoters. However, BRLF1 activates transcription of the other IE protein, BZLF1, through an indirect mechanism which we previously showed to require activation of the stress mitogen-activated protein kinases. Here we demonstrate that BRLF1 activates phosphatidylinositol-3 (PI3) kinase signaling in host cells. We show that the specific PI3 kinase inhibitor, LY294002, completely abrogates the ability of a BRLF1 adenovirus vector to induce the lytic form of EBV infection, while not affecting lytic infection induced by a BZLF1 adenovirus vector. Furthermore, we demonstrate that the requirement for PI3 kinase activation in BRLF1-induced transcriptional activation is promoter dependent. BRLF1 activation of the SM early promoter (which occurs through a direct binding mechanism) does not require PI3 kinase activation, whereas activation of the IE BZLF1 and early BMRF1 promoters requires PI3 kinase activation. Thus, there are clearly two separate mechanisms by which BRLF1 induces transcriptional activation.
Figures
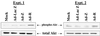







References
-
- Adamson A L, Kenney S. Rescue of the Epstein-Barr virus BZLF1 mutant Z (S186A) early gene activation defect by the BRLF1 gene product. Virology. 1998;251:187–197. - PubMed
-
- Bondeva T, Pirola L, Bulgarelli-Leva G, Rubio I, Wetzker R, Wymann M P. Bifurcation of lipid and protein kinase signals of P13K to the protein kinases PKB and MAPK. Science. 1998;282:293–296. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

