Varicella-zoster virus infection of human dendritic cells and transmission to T cells: implications for virus dissemination in the host
- PMID: 11390620
- PMCID: PMC114334
- DOI: 10.1128/JVI.75.13.6183-6192.2001
Varicella-zoster virus infection of human dendritic cells and transmission to T cells: implications for virus dissemination in the host
Abstract
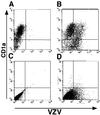
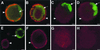
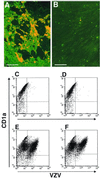
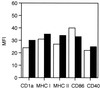
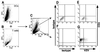
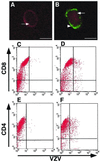
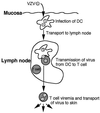
During primary varicella-zoster virus (VZV) infection, it is presumed that virus is transmitted from mucosal sites to regional lymph nodes, where T cells become infected. The cell type responsible for VZV transport from the mucosa to the lymph nodes has not been defined. In this study, we assessed the susceptibility of human monocyte-derived dendritic cells to infection with VZV. Dendritic cells were inoculated with the VZV strain Schenke and assessed by flow cytometry for VZV and dendritic cell (CD1a) antigen expression. In five replicate experiments, 34.4% +/- 6.6% (mean +/- SEM) of CD1a(+) cells were also VZV antigen positive. Dendritic cells were also shown to be susceptible to VZV infection by the detection of immediate-early (IE62), early (ORF29), and late (gC) gene products in CD1a(+) dendritic cells. Infectious virus was recovered from infected dendritic cells, and cell-to-cell contact was required for transmission of virus to permissive fibroblasts. VZV-infected dendritic cells showed no significant decrease in cell viability or evidence of apoptosis and did not exhibit altered cell surface levels of major histocompatibility complex (MHC) class I, MHC class II, CD86, CD40, or CD1a. Significantly, when autologous T lymphocytes were incubated with VZV-infected dendritic cells, VZV antigens were readily detected in CD3(+) T lymphocytes and infectious virus was recovered from these cells. These data provide the first evidence that dendritic cells are permissive to VZV and that dendritic cell infection can lead to transmission of virus to T lymphocytes. These findings have implications for our understanding of how virus may be disseminated during primary VZV infection.
Figures

References
-
- Abendroth A, Arvin A M. Varicella zoster virus immune evasion. Immunol Rev. 1999;168:143–156. - PubMed
-
- Arvin A M. Varicella zoster virus. In: Fields B N, Knipe D M, Howley P M, editors. Fields virology. New York, N.Y: Raven; 1996. pp. 2547–2585.
-
- Arvin A M, Moffat J F, Redman R. Varicella-zoster virus: aspects of pathogenesis and host response to natural infection and varicella vaccine. Adv Virus Res. 1996;46:263–309. - PubMed
-
- Arvin A M. Varicella zoster virus: virologic and immunologic aspects of persistent infection. In: Ahmed R, Chen I, editors. Persistent viral infections. New York, N.Y: John Wiley and Sons Ltd.; 1998. pp. 183–208.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

