Evidence of p53-dependent cross-talk between ribosome biogenesis and the cell cycle: effects of nucleolar protein Bop1 on G(1)/S transition
- PMID: 11390653
- PMCID: PMC87085
- DOI: 10.1128/MCB.21.13.4246-4255.2001
Evidence of p53-dependent cross-talk between ribosome biogenesis and the cell cycle: effects of nucleolar protein Bop1 on G(1)/S transition
Abstract
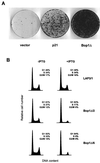
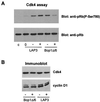
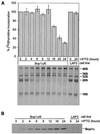
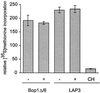
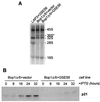
Bop1 is a novel nucleolar protein involved in rRNA processing and ribosome assembly. We have previously shown that expression of Bop1Delta, an amino-terminally truncated Bop1 that acts as a dominant negative mutant in mouse cells, results in inhibition of 28S and 5.8S rRNA formation and deficiency of newly synthesized 60S ribosomal subunits (Z. Strezoska, D. G. Pestov, and L. F. Lau, Mol. Cell. Biol. 20:5516-5528, 2000). Perturbation of Bop1 activities by Bop1Delta also induces a powerful yet reversible cell cycle arrest in 3T3 fibroblasts. In the present study, we show that asynchronously growing cells are arrested by Bop1Delta in a highly concerted fashion in the G(1) phase. Kinase activities of the G(1)-specific Cdk2 and Cdk4 complexes were downregulated in cells expressing Bop1Delta, whereas levels of the Cdk inhibitors p21 and p27 were concomitantly increased. The cells also displayed lack of hyperphosphorylation of retinoblastoma protein (pRb) and decreased expression of cyclin A, indicating their inability to progress through the restriction point. Inactivation of functional p53 abrogated this Bop1Delta-induced cell cycle arrest but did not restore normal rRNA processing. These findings show that deficiencies in ribosome synthesis can be uncoupled from cell cycle arrest and reveal a new role for the p53 pathway as a mediator of the signaling link between ribosome biogenesis and the cell cycle. We propose that aberrant rRNA processing and/or ribosome biogenesis may cause "nucleolar stress," leading to cell cycle arrest in a p53-dependent manner.
Figures



References
-
- Aktas H, Fluckiger R, Acosta J A, Savage J M, Palakurthi S S, Halperin J A. Depletion of intracellular Ca2+ stores, phosphorylation of elF2alpha, and sustained inhibition of translation initiation mediate the anticancer effects of clotrimazole. Proc Natl Acad Sci USA. 1998;95:8280–8285. - PMC - PubMed
-
- Baldin V, Lukas J, Marcote M J, Pagano M, Draetta G. Cyclin D1 is a nuclear protein required for cell cycle progression in G1. Genes Dev. 1993;7:812–821. - PubMed
-
- Bartek J, Bartkova J, Lukas J. The retinoblastoma protein pathway in cell cycle control and cancer. Exp Cell Res. 1997;237:1–6. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous
