Tissue-specific overexpression of lipoprotein lipase causes tissue-specific insulin resistance
- PMID: 11390966
- PMCID: PMC34701
- DOI: 10.1073/pnas.121164498
Tissue-specific overexpression of lipoprotein lipase causes tissue-specific insulin resistance
Abstract
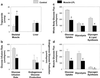
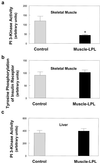
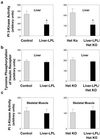
Insulin resistance in skeletal muscle and liver may play a primary role in the development of type 2 diabetes mellitus, and the mechanism by which insulin resistance occurs may be related to alterations in fat metabolism. Transgenic mice with muscle- and liver-specific overexpression of lipoprotein lipase were studied during a 2-h hyperinsulinemic-euglycemic clamp to determine the effect of tissue-specific increase in fat on insulin action and signaling. Muscle-lipoprotein lipase mice had a 3-fold increase in muscle triglyceride content and were insulin resistant because of decreases in insulin-stimulated glucose uptake in skeletal muscle and insulin activation of insulin receptor substrate-1-associated phosphatidylinositol 3-kinase activity. In contrast, liver-lipoprotein lipase mice had a 2-fold increase in liver triglyceride content and were insulin resistant because of impaired ability of insulin to suppress endogenous glucose production associated with defects in insulin activation of insulin receptor substrate-2-associated phosphatidylinositol 3-kinase activity. These defects in insulin action and signaling were associated with increases in intracellular fatty acid-derived metabolites (i.e., diacylglycerol, fatty acyl CoA, ceramides). Our findings suggest a direct and causative relationship between the accumulation of intracellular fatty acid-derived metabolites and insulin resistance mediated via alterations in the insulin signaling pathway, independent of circulating adipocyte-derived hormones.
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

