The copper transporter CTR1 provides an essential function in mammalian embryonic development
- PMID: 11391004
- PMCID: PMC34439
- DOI: 10.1073/pnas.111057298
The copper transporter CTR1 provides an essential function in mammalian embryonic development
Abstract
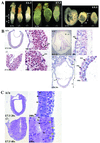
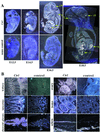
Copper serves as an essential cofactor for a variety of proteins in all living organisms. Previously, we described a human gene (CTR1;SLC31A1) that encodes a high-affinity copper-uptake protein and hypothesized that this protein is required for copper delivery to mammalian cells. Here, we test this hypothesis by inactivating the Ctr1 gene in mice by targeted mutagenesis. We observe early embryonic lethality in homozygous mutant embryos and a deficiency in copper uptake in the brains of heterozygous animals. Ctr1(-/-) embryos can be recovered at E8.5 but are severely developmentally retarded and morphologically abnormal. Histological analysis reveals discontinuities and variable thickness in the basement membrane of the embryonic region and an imperfect Reichert's membrane, features that are likely due to lack of activity in the collagen cross-linking cupro-enzyme lysyl oxidase. A collapsed embryonic cavity, the absence of an allantois, retarded mesodermal migration, and increased cell death are also apparent. In the brains of heterozygous adult mice, which at 16 months are phenotypically normal, copper is reduced to approximately half compared with control littermates, implicating CTR1 as the required port for copper entry into at least this organ. A study of the spatial and temporal expression pattern of Ctr1 during mouse development and adulthood further shows that CTR1 is ubiquitously transcribed with highest expression observed in the specialized epithelia of the choroid plexus and renal tubules and in connective tissues of the eye, ovary, and testes. We conclude that CTR1 is the primary avenue for copper uptake in mammalian cells.
Figures


Comment in
-
Mining copper transport genes.Proc Natl Acad Sci U S A. 2001 Jun 5;98(12):6543-5. doi: 10.1073/pnas.131192498. Proc Natl Acad Sci U S A. 2001. PMID: 11390990 Free PMC article. No abstract available.
References
-
- Harris E D. Proc Soc Exp Biol Med. 1991;196:130–140. - PubMed
-
- Bull P C, Cox D W. Trends Genet. 1994;10:246–252. - PubMed
-
- Danks D M. In: The Metabolic Basis of Inherited Disease. Scriver C R, Beaudet A L, Sly W S, Valle D, editors. Vol. 2. New York: McGraw–Hill; 1995. pp. 2211–2235.
-
- Vulpe C, Packman S. Annu Rev Nutr. 1995;15:293–322. - PubMed
-
- Vulpe C, Levinson B, Whitney S, Packman S, Gitschier J. Nat Genet. 1993;3:7–13. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

