Essential role for mammalian copper transporter Ctr1 in copper homeostasis and embryonic development
- PMID: 11391005
- PMCID: PMC34440
- DOI: 10.1073/pnas.111058698
Essential role for mammalian copper transporter Ctr1 in copper homeostasis and embryonic development
Abstract
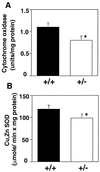
The trace metal copper (Cu) plays an essential role in biology as a cofactor for many enzymes that include Cu, Zn superoxide dismutase, cytochrome oxidase, ceruloplasmin, lysyl oxidase, and dopamine beta-hydroxylase. Consequently, Cu transport at the cell surface and the delivery of Cu to intracellular compartments are critical events for a wide variety of biological processes. The components that orchestrate intracellular Cu trafficking and their roles in Cu homeostasis have been elucidated by the studies of model microorganisms and by the characterizations of molecular basis of Cu-related genetic diseases, including Menkes disease and Wilson disease. However, little is known about the mechanisms for Cu uptake at the plasma membrane and the consequences of defects in this process in mammals. Here, we show that the mouse Ctr1 gene encodes a component of the Cu transport machinery and that mice heterozygous for Ctr1 exhibit tissue-specific defects in copper accumulation and in the activities of copper-dependent enzymes. Mice completely deficient for Ctr1 exhibit profound growth and developmental defects and die in utero in mid-gestation. These results demonstrate a crucial role for Cu acquisition through the Ctr1 transporter for mammalian Cu homeostasis and embryonic development.
Figures


Comment in
-
Mining copper transport genes.Proc Natl Acad Sci U S A. 2001 Jun 5;98(12):6543-5. doi: 10.1073/pnas.131192498. Proc Natl Acad Sci U S A. 2001. PMID: 11390990 Free PMC article. No abstract available.
References
-
- Linder M C. Biochemistry of Copper. New York: Plenum; 1991.
-
- Pena M M O, Lee J, Thiele D J. J Nutr. 1999;129:1251–1260. - PubMed
-
- DiDonato M, Sarkar B. Biochim Biophys Acta. 1997;1360:3–16. - PubMed
-
- Schaeffr M, Gitlin J D. Am J Physiol. 1999;276:G311–G314. - PubMed
-
- Valentine J S, Gralla E B. Science. 1997;278:817–818. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

