The metallochaperone Atox1 plays a critical role in perinatal copper homeostasis
- PMID: 11391006
- PMCID: PMC34441
- DOI: 10.1073/pnas.111058498
The metallochaperone Atox1 plays a critical role in perinatal copper homeostasis
Abstract
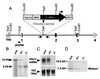
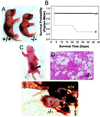
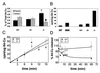
Copper plays a fundamental role in the biochemistry of all aerobic organisms. The delivery of this metal to specific intracellular targets is mediated by metallochaperones. To elucidate the role of the metallochaperone Atox1, we analyzed mice with a disruption of the Atox1 locus. Atox1(-/-) mice failed to thrive immediately after birth, with 45% of pups dying before weaning. Surviving animals exhibited growth failure, skin laxity, hypopigmentation, and seizures because of perinatal copper deficiency. Maternal Atox1 deficiency markedly increased the severity of Atox1(-/-) phenotype, resulting in increased perinatal mortality as well as severe growth retardation and congenital malformations among surviving Atox1(-/-) progeny. Furthermore, Atox1-deficient cells accumulated high levels of intracellular copper, and metabolic studies indicated that this defect was because of impaired cellular copper efflux. Taken together, these data reveal a direct role for Atox1 in trafficking of intracellular copper to the secretory pathway of mammalian cells and demonstrate that this metallochaperone plays a critical role in perinatal copper homeostasis.
Figures

Comment in
-
Mining copper transport genes.Proc Natl Acad Sci U S A. 2001 Jun 5;98(12):6543-5. doi: 10.1073/pnas.131192498. Proc Natl Acad Sci U S A. 2001. PMID: 11390990 Free PMC article. No abstract available.
References
-
- Pena M M, Lee J, Thiele D J. J Nutr. 1999;129:1251–1260. - PubMed
-
- Culotta V C, Gitlin J D. In: The Metabolic and Molecular Bases of Inherited Diseases. Scriver C R, Beaudet A L, Sly W S, Valle D, editors. Vol. 2. New York: McGraw–Hill; 2001. pp. 3105–3126.
-
- Valentine J, Gralla E B. Science. 1997;278:817–818. - PubMed
-
- O'Halloran T V, Culotta V C. J Biol Chem. 2000;275:25057–25060. - PubMed
-
- Rae T D, Schmidt P J, Pufahl R A, Culotta V C, O'Halloran T V. Science. 1999;284:805–808. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

