Myelin/axonal pathology in interleukin-12 induced serial relapses of experimental allergic encephalomyelitis in the Lewis rat
- PMID: 11395390
- PMCID: PMC1891982
- DOI: 10.1016/s0002-9440(10)64684-6
Myelin/axonal pathology in interleukin-12 induced serial relapses of experimental allergic encephalomyelitis in the Lewis rat
Erratum in
- Am J Pathol 2001 Nov;159(5):1975
Abstract
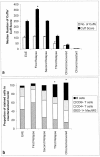
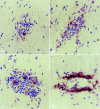
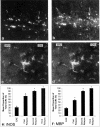
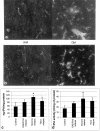
Lewis rats, on recovery from monophasic clinical experimental allergic encephalomyelitis (EAE), can be induced to develop repeated paralytic relapses with a graded reduction in clinical severity following intraperitoneal administration of IL-12. By the time of the third relapse, the number and size of inflammatory cuffs in the spinal cord were reduced with the makeup of the cellular infiltrate shifting to a significantly increased number of B cells. Serum levels of myelin basic protein (MBP)-specific IgG1 and IgG2b were found to rise over time while MBP and MBP peptide-positive macrophages and microglia became evident in perivascular cuffs and in spinal cord parenchyma, indicative of myelin phagocytosis. Axonal death was observed in semithin and EM sections of spinal cord in third relapse animals in association with iNOS and tPA immunostaining throughout gray and white matter. These neurotoxic or excitotoxic agents may contribute to axonal damage directly or indirectly by activated microglia and macrophages, leading to limited damage of the axonal-myelin unit.
Figures




References
-
- Lassman H, Zimprich F, Rossier K, Vass K: Inflammation in the central nervous system: basic mechanisms and immunological concepts. Rev Neurol 1991, 147:763-781 - PubMed
-
- Ben-Nun A, Cohen I: Experimental autoimmune encephalomyelitis (EAE) mediated by T cell lines: process of selection of lines and characterization of the cells. J Immunol 1982, 129:303-308 - PubMed
-
- Powell MB, Mitchell D, Lederman J, Buchmeier J, Zamvil SS, Graham M, Ruddle NH, Steinman L: Lymphotoxin production by myelin basic protein specific T cell clones correlate with encephalitogenicity. Int Immunol 1990, 2:539-544 - PubMed
-
- Sedgwick JD, McPhee JHM, Puklawec M: Isolation of encephalitogenic CD4+ T cell clones in the rat: cloning methodology and IFN-γ secretion. J Immunol Methods 1989, 143:3492-3497 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

