Metabolic effects of indinavir in healthy HIV-seronegative men
- PMID: 11399973
- PMCID: PMC3164882
- DOI: 10.1097/00002030-200105040-00001
Metabolic effects of indinavir in healthy HIV-seronegative men
Abstract
Background: Therapy with HIV protease inhibitors (PI) has been associated with hyperglycemia, hyperlipidemia and changes in body composition. It is unclear whether these adverse effects are drug related, involve an interaction with the host response to HIV or reflect changes in body composition.
Methods: Indinavir 800 mg twice daily was given to 10 HIV-seronegative healthy men to distinguish direct metabolic effects of a PI from those related to HIV infection. Fasting glucose and insulin, lipid and lipoprotein profiles, oral glucose tolerance (OGTT), insulin sensitivity by hyperinsulinemic euglycemic clamp, and body composition were measured prior to and after 4 weeks of indinavir therapy.
Results: Fasting glucose (4.9 +/- 0.1 versus 5.2 +/- 0.2 mmol/l; P = 0.05) insulin concentrations (61.7 +/- 12.2 versus 83.9 +/- 12.2 pmol/l; P < 0.05), insulin : glucose ratio (12.6 +/- 1.7 versus 15.9 +/- 1.9 pmol/mmol; P < 0.05) and insulin resistance index by homeostasis model assessment (1.9 +/- 0.3 versus 2.8 +/- 0.5;P < 0.05) all increased significantly. During OGTT, 2 h glucose (5.1 +/- 0.4 versus 6.5 +/- 0.6 mmol/l; P < 0.05) and insulin levels (223.1 +/- 48.8 versus 390.3 +/- 108.8 pmol/l;P =0.05) also increased significantly. Insulin-mediated glucose disposal decreased significantly (10.4 +/- 1.4 versus 8.6 +/- 1.2 mg/kg x min per microU/ml insulin; 95% confidence interval 0.6--.0;P < 0.01). There was no significant change in lipoprotein, triglycerides or free fatty acid levels. There was a small loss of total body fat (15.8 +/- 1.4 versus 15.2 +/- 1.4 kg;P = 0.01) by X-ray absorptiometry without significant changes in weight, waist : hip ratio, and visceral or subcutaneous adipose tissue by computed tomography.
Conclusions: In the absence of HIV infection, treatment with indinavir for 4 weeks causes insulin resistance independent of increases in visceral adipose tissue or lipid and lipoprotein levels.
Figures


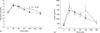
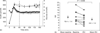
References
-
- Autran B, Carcelain G, Li TS, et al. Positive effects of combined antiretroviral therapy on CD4+ T cell homeostasis and function in advanced HIV disease. Science. 1997;277:112–116. - PubMed
-
- Cameron DW, Heath-Chiozzi M, Danner S, et al. Randomised placebo-controlled trial of ritonavir in advanced HIV-1 disease. The Advanced HIV Disease Ritonavir Study Group. Lancet. 1997;351:543–549. - PubMed
-
- Dube MP, Johnson DL, Currier JS, Leedom JM. Protease inhibitor-associated hyperglycaemia. Lancet. 1997;350:713–714. - PubMed
-
- Walli R, Herfort O, Michl GM, et al. Treatment with protease inhibitors associated with peripheral insulin resistance and impaired oral glucose tolerance in HIV-1-infected patients. AIDS. 1998;12:F167–F173. - PubMed
-
- Behrens G, Dejam A, Schmidt H, et al. Impaired glucose tolerance, beta cell function and lipid metabolism in HIV patients under treatment with protease inhibitors. AIDS. 1999;13:F63–F70. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

