Imiquimod for the treatment of genital warts: a quantitative systematic review
- PMID: 11401728
- PMCID: PMC32301
- DOI: 10.1186/1471-2334-1-3
Imiquimod for the treatment of genital warts: a quantitative systematic review
Abstract
Objective: To review published randomised controlled trials to assess the benefit and harm of imiquimod in the treatment of external genital warts.
Data sources: MEDLINE (1966 - December 2000), Cochrane Library (Issue 3, 2000) and PubMed (December 15, 2000), review articles and reference lists.
Review methods: Included studies had to be randomised trials of imiquimod, to be full published papers, and to have a comparison group. Quality of trial reporting was assessed. Relative benefit and number needed to treat were calculated for the main outcomes of wart clearance at the end of therapy, of at least 50% reduction in wart area, and of complete clearance at the end of treatment and no recurrence of warts during a follow-up period, as well as for adverse effect withdrawal or lack of efficacy withdrawal.
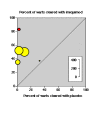
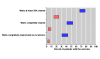
Results: There were six trials, all with quality scores of 3 (out of 5) or greater. In five trials with HIV-negative patients complete clearance of warts at the end of treatment occurred in 51% of patients treated with imiquimod 2% or 5% cream and 6% of placebo treated patients. The number needed to treat was 2.2 (95% confidence interval 2.0 to 2.6). In four trials at least 50% wart area reduction occurred with 72% of patients treated with imiquimod 5% cream and 20% of placebo treated patients. The number needed to treat was 1.9 (1.7 to 2.2). In three trials complete clearance of warts at the end of treatment plus no recurrence occurred in 37% of patients treated with imiquimod 5% cream and 4% of those treated with placebo. The number needed to treat was 3.0 (2.5 to 3.8). Adverse event withdrawal was rare and no more likely with imiquimod than with placebo. Imiquimod was not effective in one trial in HIV-positive patients.
Conclusion: The evidence base for imiquimod in treating genital warts is of high quality and the necessary size from which to draw useful conclusions. Imiquimod is effective in home application, though not in patients with HIV infection with the evidence presently available.
Figures
Comment in
-
Systematic review of imiquimod for the treatment of genital warts: all that glitters is not gold.Arch Dermatol. 2002 Dec;138(12):1599-601. doi: 10.1001/archderm.138.12.1599. Arch Dermatol. 2002. PMID: 12472350 No abstract available.
References
-
- Lamagmi TL, Hughes G, Rogers PA, et al. New cases seen at genitourinary medicine clinics: England 1998. Comm Dis Report. 1998;9 (Suppl 6):1–12. - PubMed
-
- Richwald GA, Reitano M. New approaches to the management of external genital warts. Infect Dis Clin Pract. 1999;8:67–75.
-
- Lacey C. New guidelines assist GP management of anogenital warts. Guidelines in Practice. 2001;3:25–30.
-
- Clinical Effectiveness Group (Association of Genitourinary Medicine and Medical Society for the study of Venereal Disease) National guideline for the management of anogenital warts. Sex Transm Inf. 1999;75 (Suppl 1):S71–5. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical