A novel integrin-linked kinase-binding protein, affixin, is involved in the early stage of cell-substrate interaction
- PMID: 11402068
- PMCID: PMC2192033
- DOI: 10.1083/jcb.153.6.1251
A novel integrin-linked kinase-binding protein, affixin, is involved in the early stage of cell-substrate interaction
Abstract
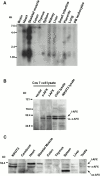
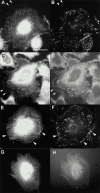
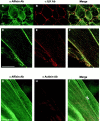
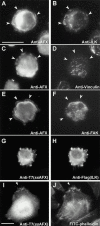
Focal adhesions (FAs) are essential structures for cell adhesion, migration, and morphogenesis. Integrin-linked kinase (ILK), which is capable of interacting with the cytoplasmic domain of beta1 integrin, seems to be a key component of FAs, but its exact role in cell-substrate interaction remains to be clarified. Here, we identified a novel ILK-binding protein, affixin, that consists of two tandem calponin homology domains. In CHOcells, affixin and ILK colocalize at FAs and at the tip of the leading edge, whereas in skeletal muscle cells they colocalize at the sarcolemma where cells attach to the basal lamina, showing a striped pattern corresponding to cytoplasmic Z-band striation. When CHO cells are replated on fibronectin, affixin and ILK but not FA kinase and vinculin concentrate at the cell surface in blebs during the early stages of cell spreading, which will grow into membrane ruffles on lamellipodia. Overexpression of the COOH-terminal region of affixin, which is phosphorylated by ILK in vitro, blocks cell spreading at the initial stage, presumably by interfering with the formation of FAs and stress fibers. The coexpression of ILK enhances this effect. These results provide evidence suggesting that affixin is involved in integrin-ILK signaling required for the establishment of cell-substrate adhesion.
Figures








References
-
- Bereiter-Hahn J., Luck M., Miebach T., Stelzer H.K., Voth M. Spreading of trypsinized cellscytoskeletal dynamics and energy requirements. J. Cell Sci. 1990;96:171–188. - PubMed
-
- Carugo K.D., Banuelos S., Saraste M. Crystal structure of a calponin homology domain. Nat. Struct. Biol. 1997;4:175–179. - PubMed
-
- Dedhar S., Williams B., Hannigan G. Integrin-linked kinase (ILK)a regulator of integrin and growth-factor signalling. Trends Cell Biol. 1999;9:319–323. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

